Oxidation Numbers
advertisement
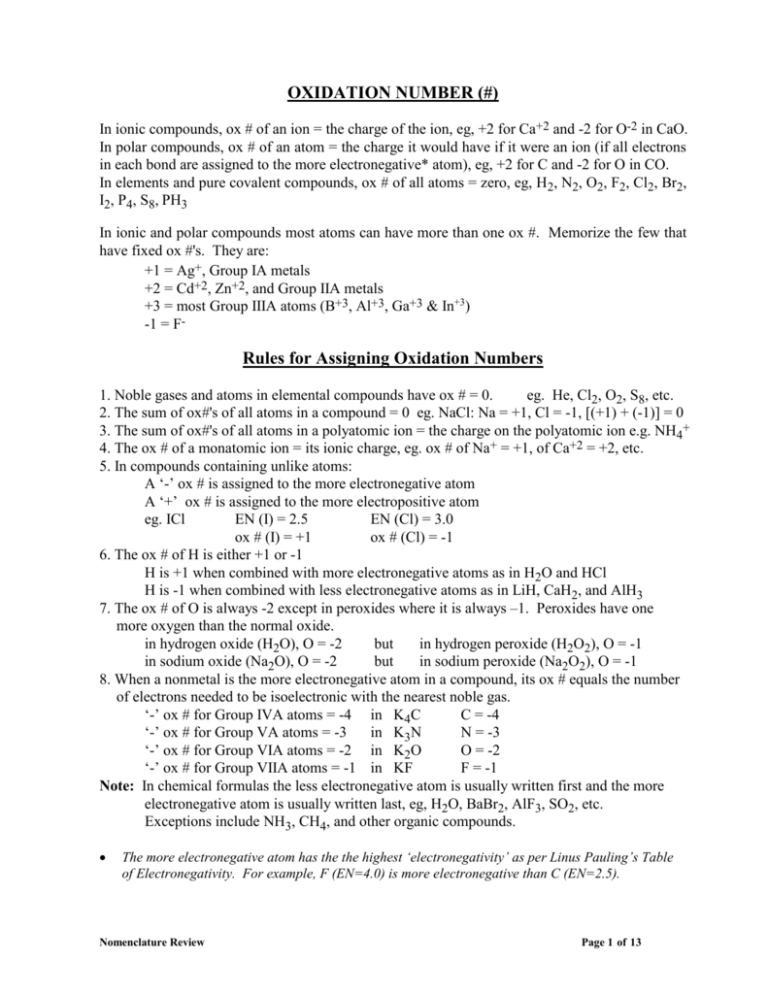
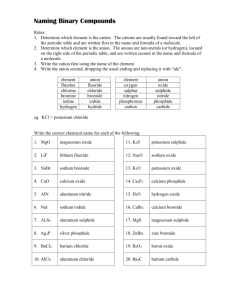
OXIDATION NUMBER (#) In ionic compounds, ox # of an ion = the charge of the ion, eg, +2 for Ca+2 and -2 for O-2 in CaO. In polar compounds, ox # of an atom = the charge it would have if it were an ion (if all electrons in each bond are assigned to the more electronegative* atom), eg, +2 for C and -2 for O in CO. In elements and pure covalent compounds, ox # of all atoms = zero, eg, H2, N2, O2, F2, Cl2, Br2, I2, P4, S8, PH3 In ionic and polar compounds most atoms can have more than one ox #. Memorize the few that have fixed ox #'s. They are: +1 = Ag+, Group IA metals +2 = Cd+2, Zn+2, and Group IIA metals +3 = most Group IIIA atoms (B+3, Al+3, Ga+3 & In+3) -1 = F- Rules for Assigning Oxidation Numbers 1. Noble gases and atoms in elemental compounds have ox # = 0. eg. He, Cl2, O2, S8, etc. 2. The sum of ox#'s of all atoms in a compound = 0 eg. NaCl: Na = +1, Cl = -1, [(+1) + (-1)] = 0 3. The sum of ox#'s of all atoms in a polyatomic ion = the charge on the polyatomic ion e.g. NH4+ 4. The ox # of a monatomic ion = its ionic charge, eg. ox # of Na+ = +1, of Ca+2 = +2, etc. 5. In compounds containing unlike atoms: A ‘-’ ox # is assigned to the more electronegative atom A ‘+’ ox # is assigned to the more electropositive atom eg. ICl EN (I) = 2.5 EN (Cl) = 3.0 ox # (I) = +1 ox # (Cl) = -1 6. The ox # of H is either +1 or -1 H is +1 when combined with more electronegative atoms as in H2O and HCl H is -1 when combined with less electronegative atoms as in LiH, CaH2, and AlH3 7. The ox # of O is always -2 except in peroxides where it is always –1. Peroxides have one more oxygen than the normal oxide. in hydrogen oxide (H2O), O = -2 but in hydrogen peroxide (H2O2), O = -1 in sodium oxide (Na2O), O = -2 but in sodium peroxide (Na2O2), O = -1 8. When a nonmetal is the more electronegative atom in a compound, its ox # equals the number of electrons needed to be isoelectronic with the nearest noble gas. ‘-’ ox # for Group IVA atoms = -4 in K4C C = -4 ‘-’ ox # for Group VA atoms = -3 in K3N N = -3 ‘-’ ox # for Group VIA atoms = -2 in K2O O = -2 ‘-’ ox # for Group VIIA atoms = -1 in KF F = -1 Note: In chemical formulas the less electronegative atom is usually written first and the more electronegative atom is usually written last, eg, H2O, BaBr2, AlF3, SO2, etc. Exceptions include NH3, CH4, and other organic compounds. The more electronegative atom has the the highest ‘electronegativity’ as per Linus Pauling’s Table of Electronegativity. For example, F (EN=4.0) is more electronegative than C (EN=2.5). Nomenclature Review Page 1 of 13 OXIDATION NUMBER EXERCISE Using the rules from the previous page, assign oxidation numbers to the underlined atoms in each compound. 1. SiF4 13. Sb2O5 2. CrI3 14. As2O3 3. CO2 15. OsO4 4. MnO4- 16. PtH4 5. CaC2 17. BaO2 6. SO3-2 18. Cu2C 7. BrO3- 19. Cr2O7-2 8. SnBr4 20. W3N5 9. Fe2O3 21. H2SiF6 10. Co2S3 22. Na2B4O7 11. P3O10-5 23. Cu3N 12. Mo3P4 24. HClO4 Answers: 1. 2. 3. 4. 5. 6. 7. 8. +4 +3 +4 +7 –1 (an exception) +4 +5 +4 Nomenclature Review 9. 10. 11. 12. 13. 14. 15. 16. +3 +3 +5 +4 +5 +3 +8 +4 17. 18. 19. 20. 21. 22. 23. 24. –1 (a peroxide) +2 +6 +5 +4 +3 +3 +7 Page 2 of 13 RULES FOR NAMING INORGANIC COMPOUNDS (The Stock System) Naming Binary Compounds (compounds made of two different atoms): 1. Think of compounds as having ‘+’ and ‘-’ atoms (even if they are not ionic). The name of the more ‘+’ atom (the atom on the left side of the periodic table) is written first. If both atoms are in the same group, the atom which is lower in the table is more ‘+’. The name of the more ‘-’ atom is written last. in NaCl sodium is more ‘+’ and chlorine is more ‘-’. 2. The name of the ‘+’ ion (cation) is the same as the element. in NaCl Na+ is called sodium (same as the element Na). If the cation has more than one possible ox #, then state its ox # in Roman Numerals. FeCl2 is named iron(II) chloride FeCl3 is named iron(III) chloride MnCl7 is manganese(VII) chloride TiCl4 is titanium(IV) chloride 3. To name the ‘-’ ion (anion), drop the ending of the element name and add the suffix "ide". SiC is silicon carbide AlP is aluminum phosphide CaO is calcium oxide KBr is potassium bromide Formulas of Binary Compounds: 1. Write the symbol of the ‘+’ ion (cation) first and the symbol of the ‘-’ ion (anion) last. Recall that the anion ends in "ide". 2. Using number subscripts, make the sum of the ox #'s in the compound = 0. It is often helpful to write the ox #'s of the ions before writing the formula of the compound. name iron(II) iodide chromium(VI) oxide zinc sulfide formula FeI2 CrO3 ZnS cation Fe+2 Cr+6 Zn+2 anion IO-2 S-2 When combining ions with more complex ox #'s, use the inverse rule, i.e., use the ox # of the one symbol as the subscript for the other symbol. +3 Al O -2 +4 Sn O -2 Al 2 O 3 Sn 2 O 4 SnO 2 Note that formulas such as Sn2O4 must be simplified to the lowest whole number subscripts. Try these. arsenic(V) oxide arsenic(III) oxide aluminum sulfide manganese(VII) sulfide magnesium carbide calcium nitride Nomenclature Review As2O5 As2O3 Al2S3 Mn2S7 Mg2C Ca3N2 THE PREFIX SYSTEM As+5 As+3 Al+3 Mn+7 Mg+2 Ca+2 O-2 O-2 S-2 S-2 C-4 N-3 Page 3 of 13 For binary compounds containing 2 non-metals, a Greek or Latin prefix is attached to the name of an element to indicate the number of atoms of that element in the compound. Number Prefix Formula Name 1 mono- CO carbon monoxide 2 di- SO2 sulfur dioxide 3 tri- SO3 sulfur trioxide 4 tetra- CCl4 carbon tetrachloride 5 penta- PCl5 phosphorus pentachloride 6 hexa- SF6 sulfur hexafluoride 7 = hepta, 8 = octa, 9 = ennea, 10 = deca, 11= hendeca, 12 = dodeca, 1/2 = hemi, 3/2 = sesqui Note: A prefix is attached to the 1st element only if more than 1 is present. Although this system is used almost exclusively for non-metal/non-metal compounds, occasionally, it is used when a metal is present. Name the following using the Prefix system and the Stock System system. Prefix System Name Stock System Name N2O dinitrogen monoxide nitrogen(I) oxide NO nitrogen monoxide nitrogen(II) oxide NO2 nitrogen dioxide nitrogen(IV) oxide N2O3 dinitrogen trioxide nitrogen(III) oxide N2O4 dinitrogen tetr(a)oxide nitrogen(IV) oxide (dimer) N2O5 dinitrogen pentoxide nitrogen(V) oxide PCl3 phosphorus trichloride phosphorus(III) chloride P2O5 diphosphorus pentoxide phosphorus(V) oxide S2Cl2 disulfur dichloride sulfur(I) choride (dimer) As2O3 (di)arsenic trioxide arsenic(III) oxide As2O5 diarsenic pentoxide arsenic(V) oxide PbO2 lead dioxide lead(IV) oxide MnO2 manganese dioxide manganese (IV) oxide U3O8 triuranium octaoxide uranium(5 1/3) oxide (not used) Fe3O4 triiron tetr(a)oxide iron(2 2/3) oxide (not used) common names include: black iron oxide or magnetite ore or magnetic iron ore Note: In the stock system, there is no space between the cation name and the oxidation number. Nomenclature Review Page 4 of 13 THE “ous-ic” SYSTEM For binary compounds in which the cation has only 2 valences, the old “ous-ic” system is sometimes used. Use the Latin name of the first element and add the suffix “ous” for the lower valency and use “ic” for the higher valency. Symbol Name Latin Name Cu copper cuprum Fe iron ferrum Pb lead plumbum Sn tin stannum Au gold aurum Name the following compounds using the “ous-ic” system. ous-ic Name Stock System Name PbCl2 plumbous chloride lead(II) chloride PbCl4 plumbic chloride lead(IV) chloride SnBr2 stannous bromide tin(II) bromide SnBr4 stannic bromide tin(IV) bromide Cu2O cuprous oxide copper(I) oxide CuO cupric oxide copper(II) oxide FeO ferrous oxide iron(II) oxide Fe2O3 ferric oxide iron(III) oxide Hg2O mercurous oxide mercury(I) oxide HgO mercuric oxide mercury(II) oxide PtCl2 platinous chloride plantinum(II) chloride PtCl4 platinic chloride platinum(IV) chloride CoO cobaltous oxide cobalt(II) oxide Co2O3 cobaltic oxide cobalt(III) oxide Ce2(SO4)3 cerous sulfate cerium(III) sulfate Ce(SO4)2 ceric sulfate cerium(IV) sulfate Nomenclature Review Page 5 of 13 OXYACIDS Oxyacids, like H2SO4 contain, in addition to a nonmetal, such as S, both hydrogen and oxygen. Students must memorize the names and formulas of the eight main oxyacids and their anions since many others are derived from these. The Eight Main Oxyacids 1 2 Acid Name Acid Formula Polyatomic Anion Formula Polyatomic Anion Name acetic acid CH3COOH or HC2H3O2 CH3COO- or C2H3O2- acetate chloric acid * HClO3 ClO3 - chlorate - bromate 3 bromic acid * HBrO3 BrO3 4 iodic acid * HIO3 IO3- iodate 5 nitric acid HNO3 NO3- nitrate H2CO3 -2 carbonate -2 sulfate 6 carbonic acid CO3 7 sulfuric acid H2SO4 SO4 8 phosphoric acid H3PO4 PO4-3 phosphate * Cl, Br and I are all in Group 7A of the periodic table and form analogous acids and polyatomic anions. Many main oxyacids also exist in forms with more and/or less oxygens. For example, a sulfur oxyacid with one less oxygen than the main acid is sulfurous acid, H2SO3. A chlorine oxyacid with one more oxygen than the main acid is perchloric acid, HClO4. In the naming system of oxyacids, ‘per…ic’ means one more oxygen than the ‘ic’ acid, ‘ous’ means one less oxygen than the ‘ic’ acid and ‘hypo…ous’ means two less oxygens than the ‘ic’ acid. A Car Never Stays Perfectly per …. ic …. ic Clean * HClO4 HC2H3O2 H2CO3 …. ous HNO3 H2SO4 H3PO4 * HClO3 HNO2 H2SO3 H3PO3 * HClO2 H3PO2 * HClO hypo …. ous Note the patterns and learn the memory aid. * Cl may be replaced by Br or I. Oxyacid anions and their salts are also named systematically. The anion of the ‘ic’ acid ends with the suffix ‘ate’. ‘per…ate’ means the anion of the ‘per…ic’ acid, ‘ite’ means the anion of the ‘ous’ acid, and ‘hypo…ite’ means the anion of the ‘hypo…ous’ acid. A Car Never Stays Perfectly per …. ate …. ate Clean * ClO4C2H3O2- …. ite CO3-2 NO3- SO4-2 PO4-3 * ClO3- NO2- SO3-2 ** HPO3-2 * ClO2- ** H2PO2- * ClO- hypo …. ite * Cl may be replaced by Br or I. ** H3PO3 has only 2 acidic hydrogens so sodium phosphite is Na2HPO3 ** H3PO2 has only 1 acidic hydrogen so sodium hypophosphite is NaH 2PO2 Nomenclature Review Page 6 of 13 SALTS OF OXYACIDS Salts of oxyacids are named using the Stock System rules like binary compounds. Formula Zn(C2H3O2) Name Formula Name zinc acetate Sn(ClO3)2 tin(II) chlorate iron(III) acetate Pb(ClO2)4 lead(IV) chlorite Cr(NO2)6 chromium(IV) nitrite Bi(ClO)5 bisthmus(V) hypochlorite Mn(NO3)7 manganese(VII) nitrate HgBrO mercury(I) hypobromite Al2(CO3)3 aluminum carbonate Co(BrO3)3 cobalt(III) bromate Ga2(SO4)3 gallium sulfate Mo(BrO4)6 molybdenum(VI) perbromate K2SO3 potassium sulfite Sr(IO2)2 strontium iodite Ba3(PO4)2 barium phosphate NaIO4 sodium periodate CaHPO3 calcium phospite LiIO lithium hypoiodite Mg(H2PO2)2 magnesium hypophosphite Be(IO3)2 beryllium iodate Cd(ClO4)2 Cu(BrO2)2 copper(II) bromite 2 Fe(C2H3O2) 3 cadmium perchlorate BINARY ACIDS Binary acids contain hydrogen and a non metal. They are all named as ‘hydro…ic’ acids when dissolved in water. In the anhydrous state (in parenthesis) they are named using standard notation. HF = hydrofluoric acid (hydrogen fluoride) HCl = hydrochloric acid (hydrogen chloride) HBr = hydrobromic acid (hydrogen bromide) HI = (hydrogen iodide) hydroiodic acid H2S = hydrosulfuric acid (hydrogen sulfide) HCN = hydrocyanic acid (hydrogen cyanide) Nomenclature Review Page 7 of 13 NAMING ACID(IC) SALTS Partially ionized polyprotic acids produce important polyatomic anions whose charges can be deduced from the original oxyacid and whose names must be memorized. The names may seem confusing at first because several conventions are used. It is important to remember that any chemical name must be unambiguous, i.e., it must be definitive, that is, each name can correspond to only one possible formula and the number of atoms must be clearly stated or must be readily determined from the name. HCO3HSO4HSO3H2PO4-1 H2PO3-1 HPO4-2 HPO3-2 H2PO3-1 H2PO3-2 (mono)hydrogen carbonate (mono)hydrogen sulfate (mono)hydrogen sulfite dihydrogen phosphate dihydrogen phosphite monohydrogen phosphate monohydrogen phosphite dihydrogen phosphite dihydrogen hypophosphite or or or bicarbonate bisulfate bisulfite Note that the prefix “bi” means ‘half’ is only applicable to acid salts of diprotic acids. Since acid salts of diprotic acids can only have one hydrogen, the prefix “mono” is optional. In the case of acid salts of triprotic acids, “bi” cannot be used and further, the number of hydrogen’s remaining must be identified by the use of “mono” or “di”. Keep in mind that in the foregoing, we are naming ions not compounds. These ions can and do form neutral compounds, called acid salts, by combining with metal cations and these are named as follows. NaHSO4 Ca(HSO4)2 Al(HSO4)3 sodium hydrogen sulfate calcium hydrogen sulfate aluminum hydrogen sulfate or or or sodium bisulfate calcium bisulfate aluminum bisulfate NaH2PO4 or sodium dihydrogen phosphate or monosodium (di)hydrogen phosphate monosodium phosphate Na2HPO4 or sodium monohydrogen phosphate or disodium (mono)hydrogen phosphate disodium phosphate For compounds of monovalent cations like Na above, i.e., Li+, K+, etc., there are at least 3 acceptable names (as shown). For polyvalent cations, like Ca+2, Al+3, etc. the convention is to state the number of H’s but not the number of metal cations, as follows. Ca(H2PO4)2 CaHPO4 calcium dihydrogen phosphate calcium monohydrogen phosphate Al(H2PO4)3 Al2(HPO4)3 aluminum dihydrogen phosphate aluminum monohydrogen phosphate Acid salts of sulfite are named like sulfate and acid salts of phosphite are named like phosphate. Nomenclature Review Page 8 of 13 Calculate the oxidation # of the underlined atom or groups of atoms in each compound. 1. N2O 11. MnO2 2. N2O5 12. Zn(HCO3) 2 3. AlPO4 13. K2CO3 -2 4. SO4 14. NO2-1 5. Cd(BrO2)2 15. SnO2 6. Ga(ClO3)3 16. AgClO4 7. SiF4 17. As2O5 8. Mg2SiO4 18. P4O10 9. Na2CrO4 10. CrO3 Answers: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. +1 +5 +5 +6 +3 +5 +4 +4 +6 +6 Nomenclature Review 19. P2O7-4 20. TiBr3 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. +4 +4 +4 +3 +4 +7 +5 +5 +5 +3 Page 9 of 13 Quiz Binary Compounds and Special Groups Write the name of formula of the following compounds. Spelling counts. The column on the right will not be marked. Formula Name Symbols & Oxid. # Cs3P Cu3N2 Mn2O7 PtCl2 SiH4 AlB Hg3As2 Cd(CN)2 Zn(OH)2 Sb2O5 nickel(III) sulfide ammonium phosphide chromium(III) oxide molybdenum(V) silicide silver cyanide gold(III) bromide gallium carbide iron(III) sulfide lead(IV) hydroxide beryllium nitride Nomenclature Review Page 10 of 13 Quiz Binary Compounds and Special Groups ANSWERS: Write the name of formula of the following compounds. Spelling counts. The column on the right will not be marked. Formula Name Symbols & Oxid. # Cs3P cesium phosphide Cs+1(only) P-3(only) Cu3N2 copper(II) nitride (Cu?)3 (N-3)2 Mn2O7 manganese(VII) oxide (Mn?)2 (O-2)7 PtCl2 platinum(II) chloride (Pt?) (Cl-1)2 SiH4 silicon hydride Si+4 (H-1)4 AlB aluminum boride Al+3(only) B-3 Hg3As2 mercury(II) arsenide (Hg?)3 (As-3)2 Cd(CN)2 cadmium cyanide Cd+2(only) [(CN)?]2 Zn(OH)2 zinc hydroxide Zn+2(only) [(OH)?]2 Sb2O5 antimony(V) oxide (Sb?)2 (O-2)5 Ni2S3 nickel(III) sulfide (Ni+3)? (S-2)? (NH4)3P ammonium phosphide [(NH4)+1(only)]? (P-3)? Cr2O3 chromium(III) oxide (Cr+3)? (O-2)? Mo4Si5 molybdenum(V) silicide (Mo+5)? (Si-4)? AgCN silver cyanide AuBr3 gold(III) bromide (Au+3)? (Br-1)? Ga4C3 gallium carbide (Ga+3)? (C-4)? Fe2S3 iron(III) sulfide (Fe+3)? (S-2)? Pb(OH)4 lead(IV) hydroxide (Pb+4)? [(OH)-1]? Be3N2 beryllium nitride [Be+2(only)]? [N-3]? Nomenclature Review Ag+(only) CN-(only) Page 11 of 13 Oxyacids & Oxyacid Salts Name ......................................... Write the name of formula of the following compounds. Spelling counts. The column on the right will not be marked. Formula Name Symbols & Oxid. # (NH4)2SO3 chromium(III) sulfate Ca(ClO)2 magnesium phosphate barium acetate Cu(BrO2)2 HBrO4 calcium iodite mercury(II) hypobromite lead(IV) chlorate manganese(VII) carbonate hydriodic acid iodic acid lithium peroxide H2SO3 magnesium bicarbonate LiHSO3 KHSO4 Na2HPO412H2O gold(II) nitrite hexahydrate HIO HBrO2 HClO3 nitrous acid sulfurous acid calcium dihydrogen phosphate aluminum monohydrogen phosphate Oxyacids & Oxyacid Salts Answers Nomenclature Review Page 12 of 13 Write the name of formula of the following compounds. Spelling counts. The column on the right will not be marked. Formula Name Symbols & Oxid. # (NH4)2SO3 ammonium sulfite SO3-2 = sulfite Cr2(SO4)3 chromium(III) sulfate (Cr+3)? (SO4-2)? Ca(ClO)2 calcium hypochlorite OCl- = hypochlorite Mg3(PO4)2 magnesium phosphate Mg+2(only), (PO4-3)only Ba(CH3COO)2 barium acetate Ba+2, CH3COO- = acetate Cu(BrO2)2 copper(II) bromite Cu+?, BrO2- = bromite HBrO4 perbromic acid Ca(IO2)2 calcium iodite Ca+2, IO2- = iodite Hg(BrO)2 mercury(II) hypobromite Hg+2, BrO- = hypobromite Pb(ClO3)4 lead(IV) chlorate Pb+4, ClO3- = chlorate (Mn)2(CO3)7 manganese(VII) carbonate Mn+7, CO3-2 = carbonate HI hydriodic acid HIO3 iodic acid Li2O2 lithium peroxide H2SO3 sulfurous acid Mg(HCO3)2 magnesium bicarbonate Mg+2, HCO3- = bicarbonate LiHSO3 lithium bisulfite Li+1, HSO3- = bisulfite KHSO4 potassium bisulfate K+, HSO4- = bisulfate Na2HPO412H2O disodium phosphate dodecahydrate Au(NO2)26H2O gold(II) nitrite hexahydrate HIO hypoiodous acid HBrO2 bromous acid HClO3 chloric acid HNO2 nitrous acid H2SO3 sulfurous acid Ca(H2PO4)2 calcium dihydrogen phosphate Ca+2, H2PO4-1 = diH phos. Al2(HPO4)3 aluminum monohydrogen phosphate Al+3, HPO4-2 = monH phos. Nomenclature Review Li+1, O-1 = peroxide Au+2, NO2-1 = nitrite Page 13 of 13