Ionic Compounds
advertisement

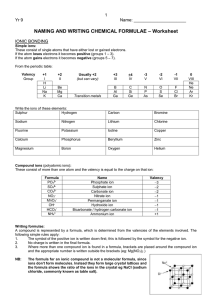
化工群專業英文校訂參考科目教學示例 建議課程綱要: 一、 元素名稱 二、 化合物命名 三、 普通化學專有名詞 四、 分析化學專有名詞 五、 基礎化工專有名詞 六、 化工裝置專有名詞 七、 儀器操作步驟 八、 實驗課程操作步驟 九、 短篇期刊文章 IUPAC Rules for Inorganic Nomenclature IONIC COMPOUNDS Positive Ions (cations) Ion Monatomic positive ions take the names of the metal from which they are derived: Name Na+ sodium ion Ca2+ calcium ion Al3+ aluminum ion When a metal forms more than one ion, it is necessary to distinguish between these ions. The accepted practice today is to indicate the charge of the ion by a roman numeral in parenthesis immediately following the name of the metal: Ion Name Fe2+ iron(II) Fe3+ iron(III) Cu+ copper(I) Cu2+ copper(II) Sn2+ tin(II) Sn4+ tin(IV) Ion The only common inorganic polyatomic positive NH4+ ions are: Hg22+ Name ammonium mercury(II) or mercurous Negative Ions (anions) Monatomic negative ions are named by adding the suffix -ide to the stem of the name of the nonmetal from which they are derived: Ion Name H- hydride F- fluoride Cl- chloride Br- bromide I- iodide O2- oxide S2- sulfide Se2- selenide Te2- telluride N3- nitride P3- phosphide C4- carbide The nomenclature of polyatomic anions is more complex. The names of the most common are: Ion Formula borate BO33- carbonate CO32- hydrogen carbonate (bicarbonate) HCO3- hypochlorite ClO chlorite ClO2- chlorate ClO3- perchlorate ClO4- chromate CrO42- dichromate Cr2O72- - cyanide CN- phosphite PO33- phosphate PO43- hydrogen phosphate HPO42- dihydrogen phosphate H2PO4- hydrogen sulfite (bisulfite) HSO3- hydrogen sulfate (bisulfate) HSO4- sulfite SO32- sulfate SO4 sulfide S2- hydrosulfide HS- hydroxide OH- nitrite NO2- nitrate NO3- oxalate C2O42- permanganate MnO4- silicate SiO4 2- 4- Ionic Compounds For ionic compounds, the name of the positive ion (cation) is given first, followed by the name of the negative ion (anion): Compound Name CaCl2 calcium chloride Fe(ClO4)3 iron(III) perchlorate FeBr2 iron(II) bromide NaHCO3 sodium hydrogen carbonate (NH4)2SO4 ammonium sulfate COVALENT COMPOUNDS For covalent compounds involving metals, the above rules are still used when metals are involved: AlCl3 aluminum chloride SnCl4 tin(IV) chloride For compounds made up of nonmetals, the first element named is the one with lower electronegativity, with the second having the higher electronegativity: Compound Name HCl hydrogen chloride H2S hydrogen sulfide NF3 nitrogen fluoride If more than one binary compound is formed by a pair of nonmetals, the Greek prefixes di (two), tri (three), tetra (four), penta (five), hexa (six), etc. are used to designate the number of atoms present. The monoprefix is rarely used. Compound Name N2O5 dinitrogen pentoxide* N2O4 dinitrogen tetroxide N2O3 dinitrogen trioxide N2O2 dinitrogen dioxide N2O dinitrogen oxide NO2 nitrogen dioxide NO nitrogen oxide *when immediately followed by a vowel, the a is dropped. Many of the most common binary nonmetal componds have common names which are use more frequently: Compound Name H2O water H2O2 hydrogen peroxide NH3 ammonia N2H4 hydrazine PH3 phosphine AsH3 arsine NO nitric oxide N2O nitrous oxide Practice Quiz.Try to name the following compounds. Compound Name ? Answers Compound Name NaF Sodium Fluoride H2S Hydrogen Sulfide K2CO3 Potassium Carbonate NaF H2S K2CO3 FeCl3 Mg(OH)2 FeCl3 Iron(III) Chloride NH4Br PbI2 Al2O3 Mg(OH)2 Magnesium Hydroxide NH4Br Ammonium Bromide CaS PbI Lead(II) Iodide CaSO3 Al2O3 Aluminum Oxide CaSO4 CaS Calcium Sulfide Ca3(PO4)2 CaSO3 Calcium Sulfite Na3N CaSO4 Calcium Sulfate Ca3(PO4)2 Calcium Phosphate Na3N Sodium Nitride Li2O Lithium Oxide K2S Potassium Sulfide NaNO3 (NH4)2S Ammonium Sulfide SO3 Fe2O3 Iron(III) Oxide Mg3P2 Magnesium Phosphide NaNO3 Sodium Nitrate SO3 Sulfur Trioxide KCl Potassium Chloride CoCl2 BaSO4 Barium Sulfate TiO ZnCl2 Zinc Chloride Li2O K2S (NH4)2S Fe2O3 Mg3P2 KCl BaSO4 ZnCl2 AgNO3 WO3 AgNO3 Silver Nitrate WO3 Tungsten (VI) Oxide CoCl2 Cobalt (II) Chloride TiO Titanium (II) Oxide