Naming Binary Compou..
advertisement

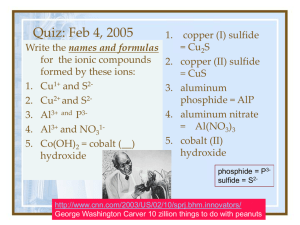
Nomenclature Naming Binary Compounds Binary Ionic Compounds Contain 2 different elements Name the metal first, then the nonmetal as -ide. Use name of a metal with a fixed charge Groups 1, 2, 13 and Ag, Zn, and Cd Examples: NaCl sodium chloride ZnI2 zinc iodide Al2O3 aluminum oxide Naming Binary Compounds Complete the names of the following binary compounds: Na3N sodium ________________ KBr potassium________________ Al2O3 aluminum ________________ MgS _________________________ Solution Complete the names of the following binary compounds: Na3N sodium nitride KBr potassium bromide Al2O3 aluminum oxide MgS magnesium sulfide Transition Metals Most form 2 or more positive ions 1+ 2+ 1+ or 2+ 2+ or 3+ Ag+ Cd2+ Cu+, Cu2+ Fe2+, Fe3+ silver ion cadmium ion copper(I) ion iron(II) ion copper (II) ion iron(III) ion Zn2+ zinc ion Names of Variable Ions Use a roman number after the name of a metal that forms two or more ions Transition metals and the metals in groups 14 and 15 FeCl3 (Fe3+) iron (III) chloride CuCl SnF4 PbCl2 Fe2S3 (Cu+ ) (Sn4+) (Pb2+) (Fe3+) copper (I) chloride tin (IV) fluoride lead (II) chloride iron (III) sulfide Naming Binary Compounds Name the following compounds: A. CaO 1) calcium oxide 2) calcium(I) oxide 3) calcium (II) oxide B. SnCl4 1) tin tetrachloride 3) tin(IV) chloride 2) tin(II) chloride C. Co2O3 1) cobalt oxide 3) cobalt trioxide 2) cobalt (III) oxide Solution Name the following compounds: A. CaO 1) calcium oxide B. SnCl4 3) tin(IV) chloride C. Co2O3 2) cobalt (III) oxide More Binary Compounds Complete the names of the following binary compounds with variable metal ions: FeBr2 iron (_____) bromide Cu2O copper (_____) oxide SnCl4 ___(_____ ) ______________ Fe2O3 ___ (_____) ______________ CuS ________________________ Solution Complete the names of the following binary compounds with variable metal ions: FeBr2 iron ( II ) bromide Cu2O copper ( I ) oxide SnCl4 tin (IV) chloride Fe2O3 iron (III) oxide CuS copper (II) sulfide Naming Binary Covalent Compounds Two nonmetals Name each element End the last element in -ide Add prefixes to show more than 1 atom Prefixes mon di tri tetra 1 2 3 4 hexa hepta octa nona 6 7 8 9 penta 5 deca 10 Naming Binary Covalent Compounds CO carbon ______oxide CO2 carbon _______________ PCl3 phosphorus _______chloride CCl4 carbon ________chloride N 2O _____nitrogen oxide Solution CO carbon monoxide CO2 carbon dioxide PCl3 phosphorus trichloride CCl4 carbon tetrachloride N 2O dinitrogen oxide Binary Covalent Compounds A. P2O5 1) phosphorus oxide 2) phosphorus pentoxide 3) diphosphorus pentoxide B. Cl2O7 1) dichlorine heptoxide 2) dichlorine oxide 3) chlorine heptoxide C. Cl2 1) chlorine 2) dichlorine 3) dichloride Solution A. P2O5 3) diphosphorus pentoxide B. Cl2O7 1) dichlorine heptoxide C. Cl2 1) chlorine (element) Binary Elements There are seven (7) elements which exist in nature as diatomic species. This means that two atoms combine to form a molecule. These elements are: hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine. Remember: HONClBrIF Binary Acids An acid is a compound that is formed between hydrogen and a second nonmetal. Examine these two compounds: HCl and HCl(aq) These compounds are named: hydrogen chloride and hydrochloric acid. Binary Acids The first compound is a typical binary compound and follows the standard binary system of nomenclature. The second compound has (aq) after the fromula which means aqueous. The compound is dissolved in water. The name for this compound is prefix = hydro (means water) add = acid (means that hydrogen is present) name = 2nd nonmetal with –ic added