Supporting information for High
advertisement
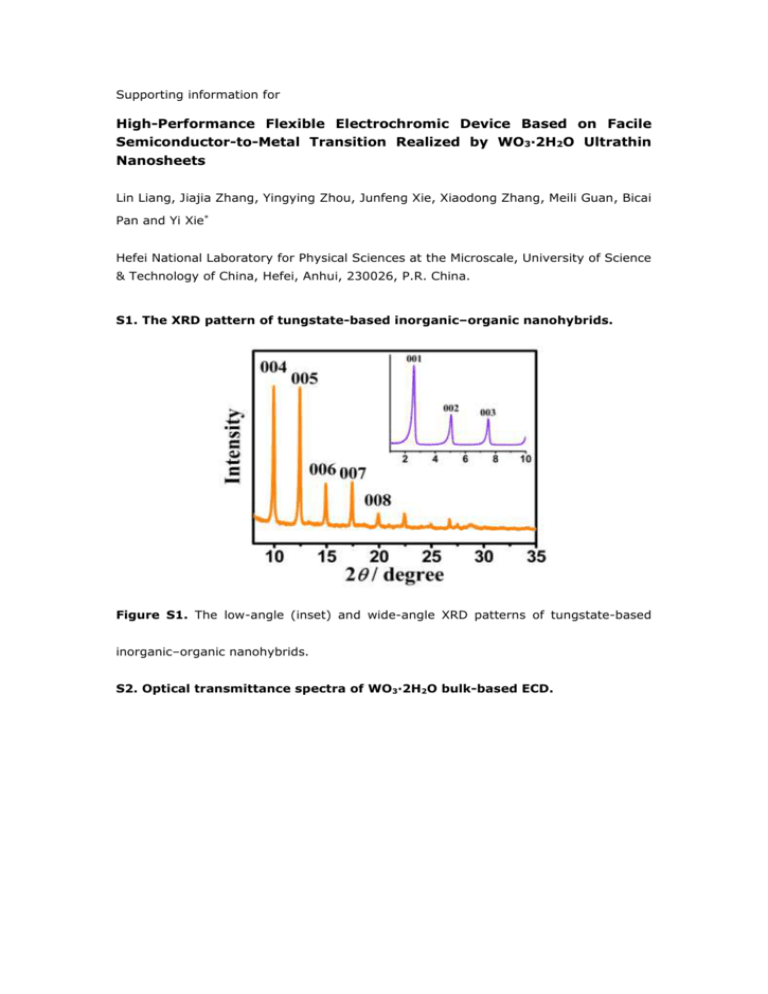
Supporting information for High-Performance Flexible Electrochromic Device Based on Facile Semiconductor-to-Metal Transition Realized by WO3·2H2O Ultrathin Nanosheets Lin Liang, Jiajia Zhang, Yingying Zhou, Junfeng Xie, Xiaodong Zhang, Meili Guan, Bicai Pan and Yi Xie* Hefei National Laboratory for Physical Sciences at the Microscale, University of Science & Technology of China, Hefei, Anhui, 230026, P.R. China. S1. The XRD pattern of tungstate-based inorganic–organic nanohybrids. Figure S1. The low-angle (inset) and wide-angle XRD patterns of tungstate-based inorganic–organic nanohybrids. S2. Optical transmittance spectra of WO3·2H2O bulk-based ECD. Figure S2. Optical transmittance spectra of WO3·2H2O bulk-based ECD under potentials of +3V and -3V, respectively S3. Density functional theory (DFT) calculations for bulk WO3·2H2O before and after Li+ intercalation. Figure S3. (a) Calculated densities of states for bulk WO3·2H2O. Calculated densities of states for bulk LixWO3·2H2O with two potential sites for Li+ intercalation: (b) between the interlamination of WO3[H2O] layer; (c) within the distorted WO5(H2O) octahedrons. S4. Detailed fabrication and characterization of tungsten oxide dihydrate (WO3·2H2O) ultrathin nanosheets film. The successful assembly and transfer of ultrathin materials into large-area thin films are very significant for device fabrication, particularly in flexible electronics. The detailed fabrication process can be summarized as below. First, the dispersion of WO 3·2H2O ultrathin nanosheets was vacuum filtrated onto a cellulose membrane with 0.22 μm pore size, forming a light-yellow homogeneous thin film, of which the thickness can be well controlled by tuning the filtrated volume of the solution; Second, the obtained WO3·2H2O thin film on cellulose membrane was pressed onto the ITO/PET sheet with the assistance of small amount of ethanol; Last, the film on the ITO/PET sheet was immersed in acetone, and the cellulose membrane can be gradually dissolved. After an hour, the acetone was aspirated and the film was reimmersed with fresh acetone again. As a result, WO3·2H2O ultrathin nanosheets film was successfully transferred onto the ITO/PET sheet. As shown in Figure S4a, the obtained WO3·2H2O films display transparent character and tunable thickness. Besides, the WO3·2H2O nanosheets on ITO/PET sheet exhibit outstanding structural integrity and flexibility as shown in Figure S4b. Figure S4. (a) Photograph of WO3·2H2O thin film with different thickness transferred onto ITO/PET sheet, showing transparent character. (b) Photograph of bend WO3·2H2O film on ITO/PET sheet, demonstrating its integrity and flexibility. S5. Preparation of WO3·2H2O bulk powder. In brief, 5 ml of 1.0 M Na2WO4·2H2O solution was dropped into 45 ml of 3 N HCl at 5 °C. A white precipitate appeared immediately, and then turned to yellow in 30 min. The final precipitate was centrifuged and washed with HCl and deionized water. The obtained yellow powder was dried for two days at room temperature. The XRD pattern of WO3·2H2O bulk powder was shown in Figure S5. Figure S5. XRD pattern for WO3·2H2O bulk powder, the orange lines give the corresponding standard pattern of JCPDS Card No.18-1420. S6. The details for first-principles density functional theory (DFT) calculations. All calculations were handled by performing density functional theory as implemented in the Vienna ab initio Simulation Package (VASP) 1. For treating the local W 5d electrons, the LDA + U 2 was employed, in which the U value of 4.0 eV was selected3. The single-particle equations were solved using the projector-augmented wave (PAW)4 method with a plane-wave cutoff of 500 eV. A vacuum space of at least 15 Å in y-axis was applied to avoid the interactions between adjacent sheets. The k-points mesh of 4×4×4 and 4×1×4 were used to sample the Brillouin zone of the bulk WO3 and nanosheets WO3. For the electronic self-consistency loop a total energy convergence criterion of 1×10-4 eV was required. Lattice constants and internal coordinates were fully optimized until residual Hellmann-Feynman forces were smaller than 0.01 eV/Å. References 1. Zhang, G. & Hewson, A. C. Non-Fermi-liquid theory of a compactified Anderson single-impurity model. Phys. Rev. B 54, 1169-1186 (1996). 2. Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505-1509 (1998). 3. González-Borrero, P. P., Sato, F., Medina, A. N., Baesso, M. L. & Bento, A. C. Optical band-gap determination of nanostructured WO3 film. Appl. Phys. Lett. 96, 061909 (2010). 4. Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758-1775 (1999).