COUNT ATOM
advertisement

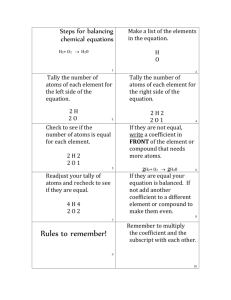
COUNT ATOM How to count atoms 2H2O COEFFICIENT SUBSCRIPT 2H2O The coefficient gets applied to the ENTIRE formula -this means that there are 2 molecules of water present! -you MULTIPLY this number by every subscript in the formula 2H2O 2MOLECULES!!!! 2H2O The subscript only gets applied to the element it follows -If there is no subscript present, it is assumed that there is only 1 atom of that element in the molecule. 2H2O How many hydrogen atoms are present? 4 How many oxygen atoms are present? 2 The easiest way to count atoms: 1. Write down all of the symbols for all of the elements present in the formula 2. If there is a coefficient, write this number next to each of the symbols 3. Multiply the coefficient by the subscripts of each of the elements present in the formula Let’s try an example: 4CaCO3 4CaCO3 1. Write down symbols Ca C O 4CaCO3 Write down the coefficient next to each symbol Ca 4 C 4 O 4 4CaCO3 Multiply by the subscript Ca 4 C O 4CaCO3 X 1 4 X 1 4 X 3 Multiply… Ca 4 C O 4CaCO3 X 1 = 4 4 X 1 = 4 4 X 3 = 12 Add totals together to get the total amount of atoms 4CaCO3 Ca 4 X 1 = 4 C 4 X 1 = 4 O 4 X 3 = 12 20 atoms 3CO2 3CO2 2C6H12O6 GLUCOSE 2C6H12O6 6K2SO4 6K2SO4 CaMg(CO3)2 CaMg(CO3)2 3Pb(NO3)2 3Pb(NO3)2