APChemistry
advertisement

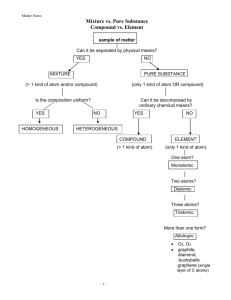
APChemistry Demonstration No. 3 Separation of a Mixture by Column Chromatography One of the most powerful tools of an analytical chemist is that of chromatography, the technique of separating and identifying the components of a mixture on the basis of the speed or distance they travel when carried along by a moving medium. For example, in paper chromatography, the mixture, generally a solution containing several components, is applied to a spot on a sheet of chromatography paper. The lower edge of the paper is dipped into a tank containing a solvent, which is carried up the sheet of paper by capillary action. As the solvent moves past the sample, the components of the sample are carried up the sheet of paper, but each component is usually carried a different distance. The distance that the component moved depends on its relative attraction for the stationary phase, the paper, and the moving phase, the solvent. The more strongly attracted the component of the mixture is to the solvent, the further up the paper the component will move. Paper chromatography can then separate the components of a mixture based on the physical property of their relative affinities for the solvent. The R f value of each component is defined as the ratio of the distance traveled by the component to the distance traveled by the solvent front [See Pg. 28 of your Textbook]. Rf Distance Traveled by Component Distance Traveled by Carrier Column chromatography is another chromatography technique in which the stationary phase is the surface of small particles of Silica Gel or other adsorbant. These particles are packed into a column by forming a slurry in a solvent such as Water. The mixture to be separated is dissolved in a the solvent and is carefully layered on top of the column. Additional solvent is placed on top of the column and allowed to carry the components of the mixture through the Stationary phase. Since components of the mixture differ in their relative attraction for the mobile phase (solvent) and the stationary phase, each component will move through the column at a different rate. The solutions containing the components can be collected as they flow off of the column. Chromatography was discovered in 1906 by the Russian botanist Mikhail Tsvett. He discovered that pigments from green plants (including chlorophyll and other compounds) could be separated by passing an ether solution of the mixture through a tube containing solid powdered Calcium Carbonate. The word "chromatography" literally means "color writing." The field was relatively dormant for the first twenty-five years, then slowly new applications and techniques were found until today there are thousands of applications and dozens of techniques including paper chromatography, Components gas chromatography, high-pressure liquid chromatography, gel electrophoresis, etc. In all types of chromatography, there is a stationary phase and a mobile phase, and the components of the mixture are separated on the basis of their relative affinity for each phase. For example, a component of a mixture that is more strongly attracted to Silica the mobile phase will move farther in a given time than a mixture that is more strongly Gel attracted to the stationary phase. Differences in the affinity of the molecules can be due to size differences, differences in polarity, volatility, stereochemistry, or almost any other atomic property. Even a simple technique such as paper chromatography can be very powerful. In fact, in the mid 1940's, Frederick Sanger was awarded to Nobel prize for chemistry based on his sequencing of the amino acids in the polypeptide Insulin. The technique that he used was paper chromatography. Modern analytical laboratories use a variety of chromatography techniques such as Gas Chromatography, High Performance Liquid Chromatography, and Gel Chromatography. No matter what the details, the basis of the technique is the relative attraction of the components of a mixture for the stationary and mobile phases. Separation of a Solution by Distillation When a solid is dissolved in a liquid (such as Copper Sulfate in Water), they form a homogeneous mixture, or a solution. The components of a solution can be separated on the basis of their different physical properties, in this case the volatility of Copper Sulfate and Water. In the process of Distillation, the solution is heated to boiling. Since the boiling point of Water is much less than that of Copper Sulfate, the Water is vaporized. Water vapor moves from the distillation flask into the condenser, where the vapor is cooled to form liquid Water again. Simple laboratory distillation apparatus. Cool water circulates through the outer portion of the condenser, causing Water vapor from the distilling flask to condense into a liquid. The non-volatile component of the mixture remains in the distilling flask. Discussion Questions: 1. What is "volatility?" Give an example of a substance that is volatile. Non-volatile. 2. What are the costs involved in distillation? 3. Can distillation be used to separate a mixture of two (or more) liquids or gases? 4. What are the practical applications of large-scale distillation of water (i.e. where might pure water be more important and much scarcer than salt water?) 5. What other separation techniques can you think of to isolate the components of a mixture? Are these based on physical or chemical properties?