DOC
advertisement

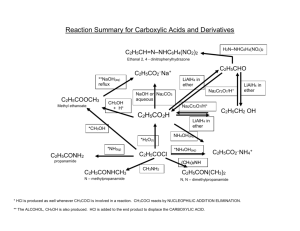
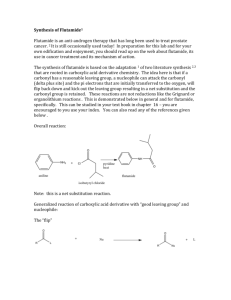
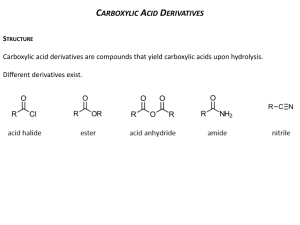
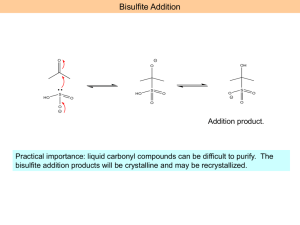
1 Chemistry 232 – Organic II Exam 2 – Dr. Gallo (Brown & Foote) November 1, 2004 Name: _____________________________ I(24) Circle the letter of the correct answer for each multiple choice question. 1.) What would result if the Grignard Reagent, reacted with H2O? A) B) C) D) E) 2.) MgBr, It would be deactivated and form 2-methylbutane. It would eliminate and form 3-methyl-1-butene. It would react to form 3-methyl-1-butanol. It would react to form 1-bromo-3-methyl-2-butanol. It would lose Mg and form 1-bromo-3-methylbutane. Which of the following sequences would not yield 3-methyl-3-hexanol O A) CH3CCH2CH3 + CH3CH2CH2MgCl O B) CH3CH2CH2 ether C CH2CH3 + CH3MgCl CH3 C) CH3CH2CH2 C CH O D) E) CH2CH3 + H2O O CH2 + H2O H3 O+ H+ ether CH3CH2MgCl + CH3C CH2CH2CH3 CH3CH2CH2C H3O+ ether H3O+ H+ CH2CH3 3.) Which of the following is the correct order of decreasing acidity (more acidic > less acidic) A) FCH2COOH > CH3COOH > F2CHCOOH B) FCH2COOH > CH3COOH > CH3OH C) CH3CH2OH > ClCH2COOH > BrCH2COOH D) CH3COOH > ClCH2COOH > CH3OH E) ICH2COOH > ClCH2COOH > FCH2COOH 2 4.) Which of the following would be a reasonable method to synthesize methyl butanoate? O H+ A) CH3CH2CH2COH + CH3OH O O B) CH3CH2CH2C O CCH2CH2CH3 + CH3OH O base C) CH3CH2CH2 C Cl + CH3OH D) All of the above E) None of the above 5.) Which statement concerning the carbonyl group is FALSE? A) Nucleophiles initially add to the carbonyl carbon for aldhyde/ketones and derivatives of carboxylic acids. B) The pi bond breaks when the nucleophile adds to the carbonyl carbon. C) A tetrahedral intermediate forms when the nucleophile adds to the carbonyl carbon. D) Protonation of the carbonyl oxygen intermediate takes place for aldehyde/ketons and derivatives of carboxylic acids. E) Nucleophilic addition takes place for aldehyde/ketons whereas nucleophilic acyl substitution results for derivatives of carboxylic acids. 6.) Which statement about the hydrolysis of a nitrile is FALSE? A) It can be carried out under acidic or basic conditions. B) The C of the C≡N is attached by the nucleophile. C) The intermediate in the hydrolysis is an amide. D) The final product of the acidic hydrolysis is a carboxylic acid. E) All of the above. F) None of the above. II(28) Complete each question in this section. 1.) 2.) Draw the structure of Z 3 methyl 2 pentenoic acid. What are the best reagents to carry out the following transformations. a) ____________________ O b) OH O OCH3 O OCH3 O C N c) C O K+ ____________________ More than 1 reagent ____________ 3 3.) 1 A compound with the molecular formula C5H10O2 gave the following HNMR spectrum: δ 0.90 δ 1.60 δ 1.95 δ 3.90 3H 2H 3H 2H t m s t IR: 1740 strong Deduce a structure consistent with this data. 4.) Aldehydes and ketones react with alcohols and an acid catalyst to form acetals. This reaction is reversible. Consider the reaction below and answer each question pertaining to it. O C H H+ + CH3CH2OH(excess) a.) What is the structure of the acetal product? ___________________________ b.) What is the structure of the intermediate in the forward and reverse reaction. c.) What is the nucleophile in the reverse reaction and where does it attack? 4 III(24) Answer both questions in this section. 1.) Show in a series of reactions how you can use the Wittig reaction to prepare the alkene molecule below. Note: Your synthesis for the organic compounds must start from an alkyl halide or alcohol. You may use whatever inorganic reagents and (C6H5)3 P as necessary. CH CCH2CH2CH3 Target CH3 2.) Provide a step by step mechanism with e‾, arrows, intermediates and products for the acid catalyzed hydrolysis of pentanamide. O CH3CH2CH2CH2CNH2 H+ H2O 5 IV(24) Complete each reaction showing the structure of the product and its stereochemistry when required. NHNH2 1.) ______________________________ + O O 2.) CH2 CH COCH3 + CH3CH2 I C C H H CrO3 3.) OH H2SO4 _______________ Pd(OAc)2 ____________________ Show stereochem K2CO3,DMF Heck Rxn. SOCl2 _______________ CH3NH2 __________ O ether + 4.) CH3CH2CH2MgCl H+ ____________________ CH3 CuI 5.) 2CH2 CHI + Li ____________ Br C C H H ______________ O 6.) C OH + CH2 * CHCH2OH __________________________________ Show position of * in product.