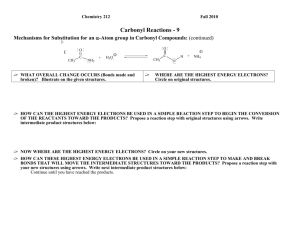
Serine and cysteine proteases
advertisement

Protease mechanisms The average person's body breaks down and resynthesizes about 450 g/day of protein. In comparison, the average person only consumes about 80 g/day of dietary protein. Obviously, there's a lot of proteolysis going on. The point of today's in-class exercise was not necessarily to get the right answers, but just to think about how an enzyme could alter the reactivity of an amide bond to speed up hydrolysis. It was great to see that almost everyone came up with perfectly reasonable mechanisms for one of the three classes of protease. I have shown the correct mechanisms below. Remember, enzymes want not merely to make a reaction happen, they want it to happen as quickly as possible. And so, they use every trick in the book to accelerate reaction rates. Serine and cysteine proteases Serine and cysteine proteases form covalent acyl-enzyme intermediates (ester, or thioester, respectively). The numbers below refer to numbers in the figures below. Serine proteases (1) In order to polarize the carbonyl C=O bond, the enzyme forms hydrogen bonds to the carbonyl oxygen. The hydrogen bond donors are NH's of either backbone or side chain (Asn, Gln) amides. This hydrogen bonding makes the carbonyl carbon more electropositive and more susceptible to nucleophilic attack. These hydrogen bonds are present throughout the mechanism, even though I've only shown them in the first step. (2) The hydroxyl group of Ser is a poor nucleophile. The nearby His side chain can act as a general base, making the Ser more nucleophilic by deprotonating it. This is an enduring mystery of Ser protease mechanisms - the pKa of His (6.5) is much lower than Ser (≈16), meaning that the concentration of R-CH2-O- should be vanishingly small. Nonetheless, nucleophilic attack does occur quickly, forming a tetrahedral intermediate. (3) This tetrahedral intermediate will not easily break down because there is no good leaving group present (pKa of an amine anion ≈ 35). So the next step is to protonate the leaving group with a general acid. Fortunately, we just formed a general acid by protonating the His side chain to give an imidazolium ion. (4) The protonated amino group can now be a leaving group, leading to breakdown of the tetrahedral intermediate. (5) Having formed an ester intermediate with the enzyme, we have to break it down using water to get the final products, R-COOH and H2N-R. Having just re-deprotonated His, we can use it as a general base to help deprotonate water, making it a better nucleophile to attack the carbonyl carbon of the ester (and don't forget the hydrogen bonds from (1)). (6) In the last step, the now re-protonated His acts as a general acid to protonate the Ser oxygen, making it a better leaving group. (N.B. Dotted lines are hydrogen bonds or Lewis acid/base interactions.) Cysteine proteases (1) Same as Ser proteases - hydrogen bonds polarize the carbonyl bond, making it more electrophilic. (2) The "resting state" of Cys proteases at physiological pH is with the Cys/His catalytic pair existing as a thiolate/imidazolium ion pair. Thiolate (R-S-) is an extremely powerful nucleophile, so the first step is pretty easy. (3) Again, we use His to protonate the tetrahedral intermediate to get the amino group to leave. (4) The tetrahedral intermediate now breaks down by departure of the protonated amino group. The enzyme has pulled a really neat trick to make sure that the C-N bond breaks rather than the C-S. In the resting enzyme, Cys is very acidic, and should be a good leaving group. However, now that His has acted as a general acid and protonated the amino group, it cannot stabilize the negative charge on the thiolate by ion pairing. Therefore, the pKa of Cys goes up and it becomes a worse leaving group than (-NH2+-R). (5) Same as Ser proteases. (6) Now that His is protonated again, it can stabilize the thiolate anion, making R-S- a good leaving group. It leaves, and we get products. A final word on Ser and Cys proteases. It may not be immediately obvious why these enzymes would break an amide bond with a two-step reaction forming a higher energy ester or thioester covalent intermediate, rather than catalyzing direct water attack on the amide bond. That's more an enzymology question than a chemistry question. Zinc proteases The key to metallo-protease mechanisms is to take advantage of the fact that Zn++ is a strong Lewis acid. We can use that strong Lewis acidity for a couple of steps. Unlike Ser and Cys proteases, the mechanism for Zn++ proteases has not been as clearly elucidated. There are two main proposed mechanisms. Both mechanisms are completely reasonable and there is experimental support for both, though the preponderance of recent evidence seems to support mechanism #1. Mechanism #1 (1) Being a strong Lewis acid, Zn++ is able to strongly polarize the carbonyl bond through a Lewis acid/base interaction with the carbonyl oxygen, making it much more electrophilic. (2) The Glu residue acts as a general base, helping to deprotonate water. (3) A water molecule acts directly as a nucleophile, aided by the strong polarization of the C=O bond and interaction with the active site Glu residue. (4) The nitrogen of the tetrahedral intermediate must be protonated to be able to leave. The proton can come from the now protonated Glu residue, or it could come from a Zn++activated water (see mechanism #2). (5) The protonated amino group now departs, and the tetrahedral intermediate collapses directly to the acid and amino products. (N.B. The solid lines emanating from Zn++ indicate interactions with other residues (usually His or Cys) in the enzyme that orient and stabilize the metal ion.) Mechanism #2 (1) In this mechanism, the protein-bound Zn++ again polarizes the carbonyl group and activates it toward nucleophilic attack. (2) The Glu carboxylate acts as a nucleophile, forming a tetrahedral intermediate. (3) Because Glu can't act as a general acid to protonate the amino group of the tetrahedral intermediate, we have to use Zn++ to activate a water molecule. A strong Lewis acid/base interaction with the lone pair electrons of a water molecule will make it more acidic and able to donate a proton to nitrogen. (4) The tetrahedral intermediate breaks down, releasing the amine product and forming an acid anhydride intermediate. (5) The Zn++-bound hydroxide generated in step (3) now acts a nucleophile, attacking the acid anhydride. This could happen at either carbonyl carbon. (6) The carboxylate of Glu is acidic and therefore a good leaving group, so the tetrahedral intermediate breaks down easily to give our products.