Functional Groups Notes
advertisement

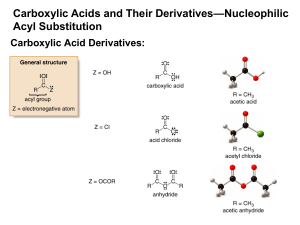
Chemistry 11 Organic Chemistry Notes - Functional Groups Definition: What is a functional group? A functional group is a sequence of atoms within a parent molecule that exhibits characteristic and predictable chemical behavior. A particular functional group generally exhibits a particular type of behavior, regardless of the nature of its parent molecule. These functional groups are molecular structural features that allow chemists to classify compounds by their reactivity. There are many different types of functional groups in organic chemistry. We will consider a subset - the functional groups which are most commonly encountered in molecules throughout the physical world. Commonly encountered functional groups Alkane Alkene Alkynes Arene Halide Alcohol Ether Amine Aldehyde Ketone Carboxylic acid Ester Amide Descriptions: what are these groups & what do they do? Alkanes, Alkenes & Alkynes Covered Arene Consist of a six-carbon hexagonal ring structure, with alternating single and double bonds between carbon molecules An example of a hydrocarbon functional group A single arene functional group is a carcinogenic molecule known as benzene Many well-known molecules contain this functional group, including steroids, cholesterol, and tetrahydrocannabinol (THC), the active chemical constituent of marijuana. 1 Chemistry 11 Halide Consist of a carbon linked to a halogen atom (F, Cl, Br, or I) Alkyl halides are insoluble in water due to the absence of hydrogen bonding (but are more polar than hydrocarbons) Alkyl halides are generally denser than water because of the relatively larger size of the halide atoms Alcohol Contain a hydroxyl (–OH) group Surprisingly, a major component of alcohols (including methanol, ethanol, and isopropyl alcohol) Ethanol (CH3CH2OH) is a clear, colorless liquid with a characteristic, pleasant odor – it is consumable in various beverages and is a component (in diluted form) of most products sold at your friendly neighborhood liquor store Methanol (CH3OH or wood alcohol) and isopropyl alcohol ((CH3)2CH2OH or rubbing alcohol) are components of household products such as windex and antifreeze. They share the characteristic odor of alcohol but, when ingested, can cause dosage-dependent negative effects ranging from permanent retinal damage to blindness to death. Isn’t it amazing the difference one carbon group more, or less, makes? Ether Consists of two carbon molecules single-bonded to a central oxygen atom The molecule ether (CH3CH2)2O, which contains the ether functional group, was used as a surgical anesthetic in the past 2 Chemistry 11 Amine Consists of a nitrogen atom single-bonded to two hydrogen atoms and one carbon atom Tend to have a fishy or ammonia-like odor Examples of common molecules containing this functional group include ammonia and methadone (used in drug rehabilitation centers) Carbonyl (includes aldehydes, ketones, carboxylic acids, esters, and amides) ALDEHYDE KETONE Carboxylic acid A member of the carbonyl family of functional groups Carboxylic acids were among the first organic compounds to be discovered Organic acids (contain carboxylic acids) tend to have a pungent odor Examples include acetic acid (vinegar ) and butyric acid (a component of rancid butter) 3 Chemistry 11 Ester A member of the carbonyl family of functional groups Molecules containing esters frequently have a fruity aroma Esters are a common component of natural and artificial flavors, including oil of wintergreen (methyl salicylate), chocolate, apple, and pineapple Amide Consists of a nitrogen atom single-bonded to two hydrogen atoms and to a carbonyl functional group (carbon double bonded to an oxygen) A member of the carbonyl family of functional groups The amide functional group is involved in the peptide bond, which links two amino acids together to form a protein 4