Enzyme Catalysis: Lab Quiz Answers
advertisement
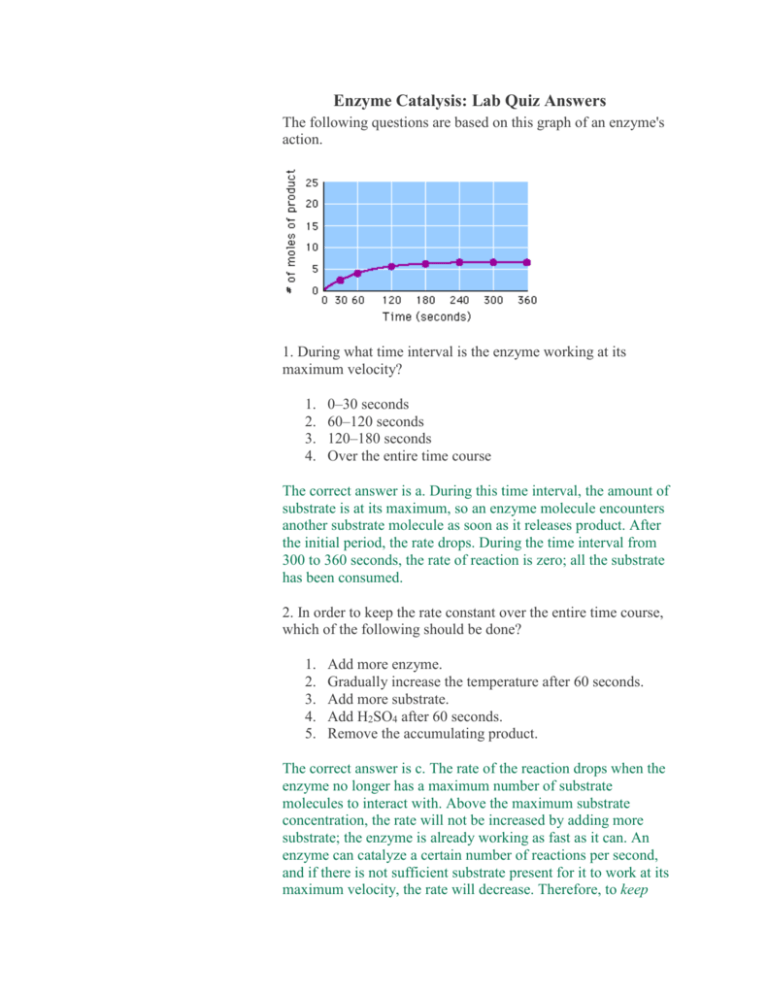
Enzyme Catalysis: Lab Quiz Answers The following questions are based on this graph of an enzyme's action. 1. During what time interval is the enzyme working at its maximum velocity? 1. 2. 3. 4. 0–30 seconds 60–120 seconds 120–180 seconds Over the entire time course The correct answer is a. During this time interval, the amount of substrate is at its maximum, so an enzyme molecule encounters another substrate molecule as soon as it releases product. After the initial period, the rate drops. During the time interval from 300 to 360 seconds, the rate of reaction is zero; all the substrate has been consumed. 2. In order to keep the rate constant over the entire time course, which of the following should be done? 1. 2. 3. 4. 5. Add more enzyme. Gradually increase the temperature after 60 seconds. Add more substrate. Add H2SO4 after 60 seconds. Remove the accumulating product. The correct answer is c. The rate of the reaction drops when the enzyme no longer has a maximum number of substrate molecules to interact with. Above the maximum substrate concentration, the rate will not be increased by adding more substrate; the enzyme is already working as fast as it can. An enzyme can catalyze a certain number of reactions per second, and if there is not sufficient substrate present for it to work at its maximum velocity, the rate will decrease. Therefore, to keep the enzyme working at its maximum, you must add more substrate. 3. Which of the following graphs represents the rate of the reaction shown in Question 1? Notice that the y-axis is the number of molecules/sec. The correct answer is B. This graph shows the rate at its maximum during the initial few seconds of the experiment. Then, as substrate is consumed, the reaction rate approaches zero. 4. What is the role of sulfuric acid (H2SO4 ) in this experiment? 1. It is the substrate on which catalase acts. 2. It binds with the remaining hydrogen peroxide during titration. 3. It accelerates the reaction between enzyme and substrate. 4. It blocks the active site of the enzyme. 5. It denatures the enzyme by altering the active site. The correct answer is e. H2SO4 lowers the pH so that the globular shape of the protein is altered. The active site is distorted to the point that the enzyme no longer functions. 5. A student was performing a titration for this laboratory, and accidentally exceeded the endpoint. What would be the best step to obtain good data for this point? 1. Estimate the amount of KMnO4 that was in excess, and subtract this from the result. 2. Repeat the titration using the reserved remaining sample. 3. Obtain data for this point from another lab group. 4. Prepare a graph of the data without this point, and then read the estimated value from the graph. The correct answer is b. The student should not throw away any remaining samples until all titrations have been completed and the data analyzed. There is sufficient sample in this lab to repeat the titration. Any other response would provide a less accurate data point.







