Atom Information
advertisement

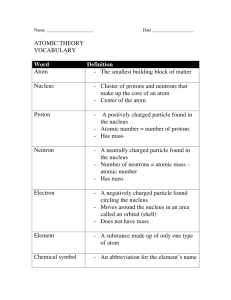
ATOMS SUMMARY NOTES on e EEM-4 Elements are pure substances that cannot be broken down into other substances by chemical or physical means. Atoms are the smallest part of an element that has the properties of that element. They are the building blocks of all matter. Elements are classified by their atomic number. The atomic number indicates the number of protons in the nucleus of an atom of that element. The number of protons equals the number of electrons. The nucleus of an atom contains positively charged particles called protons and neutral particles called neutrons. Electrons are found circling the nucleus in an area called the electron cloud. They travel close to the speed of light. Electrons have very little mass. Most of the mass of an atom is due to the protons and the neutrons, and is therefore found in the nucleus. The atomic weight of an atom is roughly equal to the number of protons plus the number of neutrons. When two or more different types of atoms combine a compound is formed. The smallest part of a compound is a molecule. Ex 1. Gold has an atomic number of 79. That means one atom of gold has 79 protons in its nucleus and 79 electrons in its electron cloud. It also has 118 neutrons in its nucleus. Therefore its atomic weight is 79 + 118 or 197. One atom of gold is the smallest amount of gold that still acts like gold. Pure gold is made up of only gold atoms. Gold cannot be broken down into other substances. Therefore gold is an element. Ex 2. The chemical formula for water is H2O. That means that the smallest amount of water that acts like water consists of two atoms of hydrogen chemically combined with 1 atom of oxygen. This is called a water molecule. There are billions of water molecules in a small amount of water. Since a water molecule is made up of several types of atoms chemically combined, water is known as a compound.