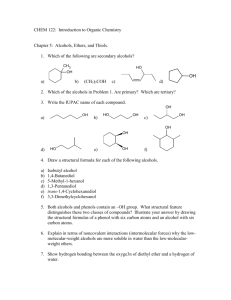
Reduction of an Alkyne to a trans Alkene
advertisement

SPRING 2008 CH221 ORGANIC CHEMISTRY 1 NOTES FOR CHAPTER 12 OXIDATION AND REDUCTION 12.1 Introduction It was shown in Chapter 4 that to tell whether an organic compound has been oxidized or reduced during a chemical reaction, it is necessary to compare the relative number of C-H and C-Z bonds (Z = a more electronegative element than C) in the starting material and product. Oxidation results in an increase in the number of C-Z (usually C-O) bonds or a decrease in the number of C-H bonds. Reduction results in a decrease in the number of C-Z (usually C-O) bonds or an increase in the number of C-H bonds. A general scheme for the oxidation and reduction of organic compounds is shown below, where replacing C-H bonds by C-O bonds is oxidation (symbol [O]), whereas the reverse is true for reduction (symbol [H]). Sometimes two carbon atoms are involved in a single oxidation or reduction reaction: in these cases, the net change of C-H or C-Z bonds at both atoms must be considered. Thus, alkynes are reduced to alkenes, which in turn, are reduced to alkanes, as shown below. 12.2 Reducing Agents Oxidation and reduction are always complementary, so that to reduce an organic compound, a substance that is itself oxidized must be used: it is called a reducing agent. Reducing agents provide the equivalent of two hydrogen atoms. There are three types of reductions considered in this chapter and these differ in how the elements of H2 are added to the organic compound being reduced. 1. Addition of molecular hydrogen. Reduction of this type is carried with a transition metal catalyst and is known as catalytic hydrogenation. 2. Addition of two protons and two electrons. Reduction of this kind is carried out by alkali metals (Li, Na or K) (these supply electrons) in liquid ammonia (which supplies the protons). They are sometimes called dissolving metal reductions. 3. Addition of a hydride (H-) and a proton. Simple hydrides (e.g. KH) can be used, but more useful are the complex metal hydrides sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4). These reagents deliver H- to the substrate and the proton comes from a protic solvent (H2O or an alcohol). 12.3 Reduction of Alkenes Alkenes are reduced by molecular hydrogen, in the presence of a solid catalyst. The process is known as (heterogeneous) catalytic hydrogenation. There is a homogeneous version (not mentioned in this chapter). The catalyst is usually a transition metal (often Pd, Pt or Ni) adsorbed onto a finely divided inert solid, such as charcoal. The catalyst 10% Pd on carbon is very widely used, but Pt is also common and oxides (e.g. PtO2) or even mixtures can be used. Catalytic Hydrogenation C C + H H weak bond tranisition metal, metal oxide or mixed catalyst C C H H Alkane Alkene The expensive catalyst can be recovered after the experiment and reused. Addition of H2 is often syn. This and other aspects of catalytic hydrogenation are demonstrated by the examples below. CH3 CH3 H2 CH3 PtO2 CH3 Illustrating stereoselectivity: often (but not always) syn addition H CH3 H Cis mainly CH3 H2 (1 equiv.) Illustrating group selectivity: only the less hindered C=C is reduced PtO2 CH3 CH3 CH(CH3)2 CH2 CH3 CH3 H2 CH3 CH3H CH3 Illustrating stereochemistry: hydrogenation occurs on less hindered side (with syn addition) Pd/C H -pinene Hydrogenation and Alkene Stability Like other alkene additions, hydrogenation reactions are exothermic because the bonds in the product are stronger than those in the starting materials. The heat of hydrogenation can be measured and used to determine the relative stabilities of alkenes. For example the heat of hydrogenation of cis-2-butene is less than that of trans-2-butene, indicating the greater stability of the latter: cis 2-Butene CH3 CH3 C H C H trans 2-Butene H2 H2 CH3CH2CH2CH3 H C Pd-C Pd-C Ho = _ 28.6 kcal/mol CH3 H C CH3 Ho = _ 27.6 kcal/mol More stable starting material, less heat released The Mechanism of catalytic Hydrogenation It is generally accepted that adsorbed atoms and molecules react on the surface of the catalyst, having been activated by it. The hydrogen atoms are transferred sequentially to the alkene to produce the alkane, which is then desorbed, because there is no longer a metal- bond attraction. The above mechanism is able to explain several features of catalytic hydrogenation shown in the examples earlier: Syn addition (though this is not 100% reliable). Less hindered C=C reacts more readily than hindered C=C. Addition occurs on less hindered side of alkene. Hydrogenation Data and Degrees of Unsaturation In Chapter 10, it was shown that the number of degrees of unsaturation gives the total number of rings and bonds in a molecule. Hence hydrogenation can enable the determination of the number of bonds and rings by comparison of the number of degrees of unsaturation before and after hydrogenation. This is illustrated by Sample Problem 12.1. Hydrogenation of Other Double Bonds Other unsaturated systems can be reduced using catalytic hydrogenation. Sometimes these compete successfully with C=C bond reduction (e.g. –COCl and NO2). Others, such as aldehydes, carboxylic acids, esters and ketones react more slowly than alkene functions, so that C=C can often be reduced in the presence of, say, a ketone function, with the latter being unaffected. Benzene rings are hydrogenated only with difficulty, requiring the use of a Raney nickel catalyst. 12.4 Application: Hydrogenation of Oils Partial hydrogenation of the unsaturated long chain fatty acid chains of the triacylglyceride molecules in vegetable oils is used to prepare many processed foods, such as cake, cookies, margarine, and peanut butter. The fewer double bonds in the triacylglycerols result in a higher melting point (m.p.) of the lipid, thus giving a material of semi solid consistency (a low melting point solid). The greater the extent of hydrogenation, the higher the m.p. of the resulting material. Hence it is possible to produce hard and soft fats by this process, which is commercially known as hardening. Hydrogenation increases the shelf life of the lipid by lowering the number of allylic carbon atoms. These carbon atoms are most susceptible to oxidation that causes off-flavors (rancidity). 12.5 Reduction of Alkynes An alkyne can be reduced either to the alkene with one equivalent of the elements of H2 or to the alkane by using two equivalents of the elements of H 2. In the case of partial reduction to the alkene, the elements of H2 may be added in a syn or anti fashion, depending on the catalyst. syn Addition gives cis alkene H2 (1 equiv) catalyst R R C C H R C C H one further equiv of H2 R R H2 (1 equiv) catalyst H C H C R H H C C H H R Alkane R anti Addition gives trans alkene Reduction of an Alkyne to an Alkane Reaction of an alkyne with two equivalents of molecular hydrogen, in the presence of a catalyst (as for alkene hydrogenation) results in the formation of the corresponding alkane. When normal catalysts (e.g. 10% Pd-C) are used, syn addition occurs to give the alkene, which is then further reduced to the alkane. The alkene is not isolated and indeed, normal hydrogenation catalysts are too reactive to allow hydrogenation to stop at the alkene: if an alkene product is required, special techniques are needed, as described next. Reduction of an Alkyne to a cis Alkene Alkynes can be hydrogenated to cis alkenes by the use of a partially poisoned (deactivated) catalyst, known as a Lindlar catalyst: H2 R C C R R R C Pd on CaCO3 with added lead acetate and quinoline (Lindlar catalyst) C H H cis Alkene This reaction is more or less specific for alkyne triple bonds and the product alkene is unreactive toward further hydrogenation under these conditions. An example is given in Problem 12.10. C=O and C=C unaffected O O CH2 CH3 C C CH2CH3 H2 CH2 H Lindlar catalyst C syn addition of one equivalent of H2 C CH2CH3 H CH3 cis-Jasmone (aroma component of jasmine flower) Reduction of an Alkyne to a trans Alkene Complementary to the above technique, it is possible to partially reduce an alkyne to the trans alkene. This time hydrogenation is not used, instead a dissolving metal reduction delivers the elements of H2 in an anti fashion. The mechanism of dissolving metal reduction (sometimes known as Birch reduction) for Na in NH3 is shown below. It involves sequential electron transfers (from Na to organic species) and proton transfers (from NH3 to organic species) and includes the formation of a radical anion, a radical and a carbanion. _ e (from Na) R1 C C R2 . C R1 _ .. C R2 a radical anion _ .. C . C R1 . C R1 H R2 R2 NH2 . C R1 _ -NH2 R2 C H a radical _ e (from Na) R2 _ .. C C H C H R1 an anion (in preferred trans configuration) H _ .. C NH2 R2 C H R1 H R2 C _ -NH2 C R1 H trans alkene In the 3rd step, the carbanion is formed preferentially in a trans configuration, thus giving trans stereoselectivity to the whole process. The trans carbanion is sterically less hindered than the cis isomer. H R C R H C .._ More stable vinyl anion C R _ .. C R Unfavorable steric interactions destabilize this vinyl anion (which would give cis alkene) trans Alkene 12.6 Reduction of Polar C-X Bonds Compounds containing polar C-X bonds that react with strong nucleophiles can be reduced with metal hydride reagents, especially lithium aluminum hydride. Two common functional groups that have both these characteristics are alkyl halides and epoxides. Reduction of these C-X bonds is another example of nucleophilic substitution and because H- is a strong nucleophile, the reaction follows an SN2 mechanism. _ H3Al R H + CH2 R SN2 X _ H CH2 + X + AlH3 LiAlH4 donates H- to electrophilic C Two examples are given below, illustrating the easier reduction of 1o alkyl halides and the orientation of hydride transfer from AlH4- to the epoxide. 12.7 Oxidizing Agents Oxidizing agents are broadly of two types: Reagents that contain an oxygen-oxygen bond. Reagents that contain metal-oxygen bonds. Oxidizing agents with O-O bonds include O2, O3 (ozone), H2O2 (hydrogen peroxide), (CH3)3COOH (tert-butyl hydroperoxide) and RCO3H (peroxyacids). Of these, peroxy acids are the most widely used: examples are shown below. O O C R O OH CH3 O O C C O C O O OH OH _ CO2 Peroxyacetic acid Peroxyacid Cl meta-chloroperoxybenzoic acid mCPBA OH Mg2+ 2 Magnesium monoperoxyphthalate MMPP The two most common oxidizing agents that contain metal-oxygen bonds are based on Mn(VII) and Cr(VI). Potassium permanganate (KMnO 4) is the most usual Mn(VII) reagent, used in acidic, neutral or alkaline conditions, depending on the substrate to be oxidized and the desired degree of oxidation. Cr(VI) reagents include chromium (VI) oxide, and acidified sodium or potassium dichromate (H+/Cr2O72- or H2SO4/K2Cr2O7)). These are all strong oxidizing agents with little selectivity. Much more selective is pyridinium chlorochromate (PCC), which can be used in chlorinated organic solvents (e.g. CH2Cl2), without strong acid. 6+ oxidation state 6+ oxidation state O O _ Cr O + N O Chromium (VI) oxide CrO3 O Cr Cl O H Pyridinium chlorochromate PCC The types of oxidation of alkenes, alkynes and alcohols that can be performed using one or other of the above oxidizing agents is shown below and is the main topic for the rest of Chapter 12. 12.8 Epoxidation Epoxidation involves the addition of a single oxygen atom to an alkene to form an epoxide. O O C + m-ClC6H4 O OH C O + m-ClC6H4 mCPBA The mechanism is a concerted addition of O to the alkene bond, rather like the first step in halogenation that forms the bridged halonium ion intermediate. .. O: R R C The peroxide (O-O) bond is broken = .. O: C :O .. H .. O .. C C .. O: R C H :O .. O C H :O .. .. O .. C C C OH Stereochemistry of Epoxidation The concerted mechanism above requires syn addition of O to the double bond thus leading to retention of configuration (a cis alkene gives a cis epoxide). The addition can occur from either above or below the plane of the double bond, giving the reaction a stereospecific character, as illustrated for the epoxidation of cis- and trans-2-butenes, below. Attack from below Attack from above CH3 CH3 O mCPBA C C H H cis-2-Butene CH3 H C * C* + CH3 H CH3 H * C C* CH3 H O Achiral meso compound CH3 H C C H mCPBA CH3 Attack from below Attack from above O CH3 H C* C* H CH3 + CH3 H * C * C H CH3 O trans-2-Butene Enantiomers The Synthesis of Disparlure Disparlure, the sex pheromone of the female gypsy moth is synthesized by a sequence of steps, the last of which is an epoxidation reaction. The synthesized compound (as a cheap racemate) is mixed with a sticky substance and used as a lure or trap for male moths. The males can detect single molecules of disparlure and flock to the source of the pheromone, where they become stuck in the sticky substance and eventually die. This is an example of a pheromone trap – a way to kill insect pests without the need for an environmentally unfriendly insecticide. The gypsy moth has been responsible for the deaths of many broadleaf trees: it has a voracious appetite for the leaves. The retrosynthetic analysis and actual synthesis of disparlure are shown next. 12.9 Dihydroxylation (or Perhydroxylation) Dihydroxylation is the addition of two hydroxy groups (the elements of H2O2) to a double bond to form a 1,2-diol (glycol). This is illustrated for cyclohexene below, where it can be seen that choice of reagent is important in deciding the major diasteroisomerism of the product (i.e. whether it is cis or trans). 1. RCO3H 2. H+(aq) or OH-(aq) OH anti Addition OH OH syn Addition Either KMnO4/OH-(aq) or 1. OsO4 2. NaHSO3(aq) OH The epoxidation of cyclohexane followed by hydrolysis route leads to trans-1,2cylclohanediol. This can be explained by the necessity of a backside attack by H2O on the protonated (activated) epoxide, so that the two C-O bonds are formed on opposite sides of the ring. OH Attack from below at C2 OH .. : OH2 RCO3H : O: Cyclohexene H+ 2 1 + :O -H+ Racemic transcyclohexane-1,2-diol H .. : OH2 -H+ Attack from below at C1 OH OH In practice, racemic trans-1,2-cyclohexanediol is formed, illustrating the principle that an achiral substrate plus achiral reagent give either an achiral or a racemic product. In contrast, alkaline KMnO4 or OsO4 followed by aqueous NaHSO3 convert cyclohexene to cis-1,2-cyclohexanediol. O O O O O Os + O .. Os O NaHSO3/H2O O OH OH Note the syn addition cis-Cyclohexane-1,2-diol _ O O pH > 7 O Mn + O O O .. Mn O _ H2O O Potassium permanganate is cheap and relatively nontoxic, but is not soluble in organic solvents (although it can be used with phase transfer catalysts). Osmium tetroxide is more expensive and highly toxic, but is soluble in organic solvents and is more selective than KMnO4. N-methyl morpholine N-oxide, with a catalytic amount (a few %) of OsO4 can be used in place of OsO4 itself. _ CH3 + N O N-Methylmorpholine N-oxide The N-methylmorpholine N-oxide first oxidizes Os(VI) to Os(VIII), which then adds O to the alkene double bond and is reduced back to Os(VI). This cycle continues throughout the reaction. 12.10 Oxidative Cleavage of Alkenes This involves complete rupture (breaking both and bonds) of the double bond to form two carbonyl groups – aldehydes or ketones, depending on the extent of substitution at each alkene C atom. OH OH The most common way of doing this is by ozonolysis: oxidative cleavage with ozone, followed by treatment with either Zn and water or dimethyl sulfide. The mechanism of ozonolysis involves addition of O 3 to C=C as the first step. The initial addition product then undergoes rearrangement to an ozononide which is then reductively cleaved by Zn/H2O (older method) or (CH3)2S to afford carbonyl compounds. Sample Problem 12.3 shows two examples: the second part illustrates the usefulness of ozonolysis in preparing ,-dicarbonyl cycloalkenes. Otherwise the technique is synthetically limited. compounds from Ozonolysis was once a good method for the determination the locations of double bonds in unknown molecules: an example is shown below for the terpene limonene and also in Sample Problem 12.4. 12.11 Oxidative Cleavage of Alkynes Alkynes undergo ozonolysis to give carboxylic acids and CO 2, depending on whether the alkyne C atoms are internal or terminal. 12.12 Oxidation of Alcohols Alcohols can be oxidized to a variety of carbonyl compounds, depending on the type of alcohol (1o, 2o or 3o) and the reagent. Oxidation occurs by replacement of C-H bonds on the carbon bearing the OH group with C-O bonds. Cr(VI) reagents are the usual oxidizing agents: CrO3, Na2Cr2O7 and K2Cr2O7 are vigorous, nonselective reagents that require aqueous acid. PCC is milder and more selective, is soluble in CH2Cl2 and does not require acid. Oxidation of 2o Alcohols Any of the above Cr(VI) reagents effectively oxidize 2o alcohols to ketones by the following mechanism. The first step is formally addition of the alcohol across one of the Cr=O bonds. This produces an alkyl chromate ester, with the Cr atom still in oxidation state +6. The ester is then decomposed by hydrogen abstraction to water, forming the carbonyl bond and the Cr atom being reduced to Cr(IV). By a subsequent series of steps (not shown) Cr(IV) is further reduced to the green Cr(III). Step 1 Formation of chromate ester O R2CH .. OH .. Cr + O (VII) (with proton transfer) O (VII) R2CH O O Cr OH O Chromate ester Step 2 Proton abstraction from the chromate ester to form the carbonyl group O (VII) R2C H O Cr (IV) OH O .. H2O: R2C O _ CrO3H H + H2O: Oxidation of 1o Alcohols Primary alcohols are oxidized to aldehydes or carboxylic acids depending on the reagent: CrO3 (etc) oxidizes them all the way through to carboxylic acids, whilst the milder PCC produces aldehydes. The mechanism of oxidation of 1o alcohols using CrO3 or similar reagents involves the same features of the mechanism just discussed for 2 o alcohols. The major difference is that once the aldehyde is formed in the first part, it easily forms a hydrate in the aqueous environment of the reaction, thereby creating new OH bonds that can form chromate esters with excess Cr(VI), resulting in further oxidation (parts 2 and 3). Part 1 Oxidation of 1o alcohol to aldehyde H R R C OH + Cr(VI) C O + H H Cr(IV) By mechanism for oxidation of 2o alcohols, described previously Aldehyde 1o Alcohol Part 2 Addition of H2O to aldehyde to form hydrate H H+ R C O R H2O + H C OH OH Hydrate (a 1,1-diol) Part 3 Oxidation of hydrate to carboxylic acid .. H2O: H H CrO 3 R C OH R OH two steps C OH O O R Cr O Chromate ester OH C O + .. _ CrO3H + .. + H3O HO Carboxylic acid Also similar to the mechanism described for the oxidation of 2o alcohols described previously The oxidation of alcohols by orange Cr(VI) reagents is accompanied by a color change to green as Cr(VI) is reduced to Cr(III). This was the basis of the first “on the spot” alcohol testing devise, used by police on drivers suspected of having over the legal limit of ethanol in the blood stream. The individual is required to blow into a tube containing K2Cr2O7 and H2SO4 on an inert substance. The exhaled gases are forced through the tube and turn the crystals green to a depth that depends on the amount of ethanol in the breath (and hence in the blood stream). 12.13 Green chemistry Organic chemists have long been seeking safer, more environmentally friendly ways of performing syntheses, including those that involve oxidation. In particular, interest is growing on reactions in water, solventless reactions (or those that require less solvent) and reactions that use milder, less dangerous reagents (e.g. enzymes or microorganisms, like baker’s yeast) and produce either safe byproducts or those that can be removed from the reaction mixture by simple physical processes (such as filtration). In the realm of Cr(VI)-catalyzed oxidation of alcohols, polymer supported CrO3 fulfills some of the characteristics mentioned above. Chromium oxide in acidic solution exists partially as hydrogen chromate. This can be supported on an ion-exchange resin such as the strongly basic macroporous Amberlyst A-26 resin (a cross-linked polystyrene-divinylbenzene copolymer with quaternary ammonium ((CH3)3N+groups) by ion exchange. _ HCrO4 (from CrO3(aq)) P _ + N(CH3)3 Cl -Cl Amberlyst A-26 resin (as purchased) P _ + N(CH3)3 HCrO4- "Polymer-supported CrO3" Stir CrO3 (aq) with Amberlyst A-26, filter and dry This reagent is milder than the standard Cr(VI) reagents (see below) and the Cr(III) residue can be filtered off with the spent polymer after the reaction. The polymer can be regenerated. Ph2CH OH HCrO4Amberlyst A-26 Ph2C O HCrO4Amberlyst A-26 CH3(CH2)8CH2OH CH3(CH2)8CHO 12.14 Application: Oxidation of Ethanol Ingested ethanol is metabolized in the liver by oxidation, first to ethanol and finally to acetate. The enzyme performing this oxidation is liver alcohol dehydrogenase (LAD), a protein that works with the redox coenzyme known as nicotinamide adenine dinucleotide (NAD+ + H+ + 2e- NADH). If more ethanol is ingested than can be metabolized in a given time, the concentration of the toxic acetaldehyde builds up and leads to the condition known as a hangover. Antabuse is a drug given to alcoholics to stop them consuming alcohol. The drug allows the metabolism of ethanol to acetaldehyde to occur, but inhibits the oxidation of acetaldehyde to acetate, thus causing the subject to become violently ill. Methanol (often a minor component of alcoholic beverages) is also metabolized by liver alcohol dehydrogenase to the even more toxic formaldehyde and formic acid. However, it is metabolized more slowly than ethanol, so that in cases of methanol poisoning, ethanol is administered to the patient to switch the action of LAD from methanol to ethanol. The unmetabolized methanol is then harmlessly excreted unchanged. 12.15 Sharpless Epoxidation It has been demonstrated in several previous chapters that an achiral starting material reacts with an achiral reagent to give either an achiral product or a racemic mixture of two enantiomers. In this chapter we have seen that the synthesis of disparlure gives a racemic product, where only one of the enantiomers is physiologically active. A reaction that produces predominantly or exclusively one enantiomer is known as an enantioselective reaction: if it converts an achiral starting material to predominantly one enantiomer, it is also an asymmetric reaction. The Sharpless epoxidation (devised by K B Sharpless in the 1980s) is an aymmetric reaction that oxidizes allylic alcohols to epoxides. The reagent is tBuOOH, with pure (-) or (+) diethyl tartrate and titanium (IV) isopropoxide providing the chiral influence. The configuration of the epoxide product can be predicted according to whether (-)- or (+)-DET are used, as shown below. Sample problem 12.5 serves to provide a couple of examples of Sharpless epoxidation, along with prediction of epoxide configuration.