5-3 Periodic Trends
advertisement

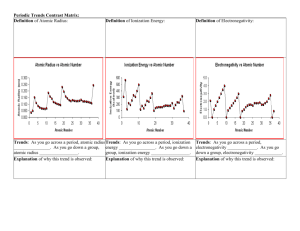
PERIODIC TRENDS Atomic Radius The bond lengths and atomic radii for selected substances are provided in the table below. Substance Bond Length (Å) Atom Atomic Radius (Å) F2 (F–F) 1.4119 F 0.70595 Cl2 (Cl–Cl) 1.9878 Cl 0.99390 Br2 (Br–Br) 2.2811 Br I2 (I–I) 1.3332 Na2 (Na–Na) 3.0789 Na 1.5395 Si2 (Si–Si) 2.246 Si 1.123 P2 (P–P) 1.8931 P S2 (S–S) I S 0.94460 According to the table above, atomic radius is defined as __________ the distance between the _______________ of identical atoms that are _______________ together. 1. Calculate the atomic radius of bromine and phosphorus. 2. Calculate the bond length of iodine (I2) and sulfur (S2). What’s the trend? Trend explanation: o Period Trend What happens to the size of the outer shell as you move left to right across a period? Remember, electrons are filling the sublevels within the same energy level… What happens to the number of protons in the nucleus as you move left to right across a period? So if the nucleus is getting _______________ and the outer shell is not getting _______________, what will happen to the radius as you go across the period? o Group Trend What happens to the size of the outer shell as you move down a group? So if the outer shell is getting _______________, what will happen as you go down the group? Ionic Radius An ion is simply defined as a _______________ particle. o A positively charged ion forms from _______________ electrons, so as the remaining electrons draw closer to nucleus because the number of protons was not affected, the radius _______________. o Metals tend to form _____ charged ions called _______________. Neutral atom __________ o A negatively charged ion forms from _______________ electrons. This causes the electron cloud to expand, which causes the radius to _______________. o Nonmetals tend to form _____ charged ions called _______________. Neutral atom __________ What’s the trend? o Same as _______________ _______________, but now with charged particles instead of neutral atoms. Electronegativity Measure an atom’s ability in a chemical compound to __________ electrons. Trend explanation: o Group trend: Fluorine has __________ valence electrons, which means it is only _____ electron away from being as stable as a Noble Gas. It is desperate for that electron, so it has a _______________ attraction for electrons. Therefore, it has a __________ electronegativity. Lithium has _____ valence electron, which means it is also only _____ electron away from being as stable as a Noble Gas. It is desperate to __________ its outer electron, so it has a _______________ attraction for electrons. Therefore, it has a __________ electronegativity. o Period trend: We’ve already established that fluorine has a high electronegativity, but there’s another reason for this: Its valence shell is only n = 2, so it is rather __________ to the nucleus. The stronger nuclear pull also helps fluorine have a high electronegativity. Astatine has __________ valence electrons, so it is also desperate for that last electron to fill its shell, but since its valence shell is n = 6, the pull of the nucleus on electrons is weaker than fluorine’s. Therefore, it has a __________ electronegativity. What’s the trend? Fluorine has the _______________ electronegativity - assigned _____ by Linus Pauling’s relative electronegativity scale. The values of other element electronegativities have been calculated in relation to Fluorine’s. Cesium and francium share the _______________ electronegativity at _____. Electron Affinity The _______________ change that occurs when an electron is _______________ from a neutral atom Most atoms _______________ energy when they acquire an electron, so most affinities are negative. Trend explanation: o Period trend: As the p–orbital is filling, the element tends to want electrons more (to fill their outer shells), so electron affinity gets __________ _______________ to the right. o Group trend: As you go down a group, the pull of the nucleus on electrons gets _______________, so electron affinity gets __________ _______________ down a group. What’s the trend? Ionization Energy Ionization: Any process that results in the formation of an __________. Ionization Energy: The energy required to _______________ one electron from a neutral atom. Trend explanation: o Period trend: As you go across a period, the nucleus _______________ in strength, making it _______________ to remove electrons from the atoms. Therefore, ionization energy _______________. o Group trend: As you go down a group, the electrons are getting _______________ from the nucleus, making it _______________ for the electron to be removed. Therefore, ionization energy _______________. What’s the trend? Metallic and Nonmetallic Character Metal, nonmetal and metalloid characteristics were discussed in chapter 1. What’s the trend? Metals are on _______________ side of the “_____-_____” line, nonmetals are on _______________ side of the “_____-_____” line Metalloids surround the “_______________” line – only _____ of them! Reactivity Reactivity depends on the number of _______________ electrons the element has. o It also depends on the reactivity of the other _______________, so this is a general reactivity trend. We will discuss metals and nonmetals separately. Metals: o The _______________ valence electrons they have, the _______________ reactive they are. o Trend explanation: Group Trend: Electrons are not held as strongly as the atomic radius increases, so it’s _______________ to remove electrons further down the group. Reactivity _______________. Period Trend: Electrons are held more tightly as the nuclear charge increases, so it’s _______________ to remove electrons across a period. Reactivity _______________. What’s the trend? Nonmetals: o The _______________ valence electrons they have, the _______________ reactive they are. Trend explanation: o Group Trend: p-sublevels that are close to the nucleus can be filled up _______________ because the nucleus has a _______________ pull on free electrons. Reactivity _______________. o Period Trend: As the _____-sublevel is being filled up, the elements tend to want the electrons more because they’re getting closer to being _______________. Reactivity _______________. What’s the trend?