Supplementary Figure Legends (doc 46K)
advertisement

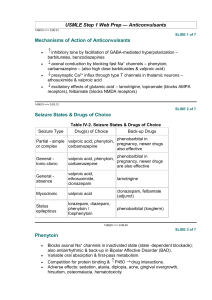
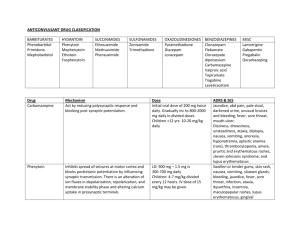
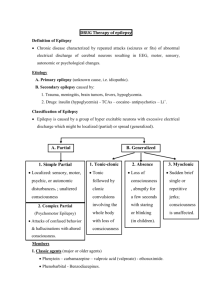
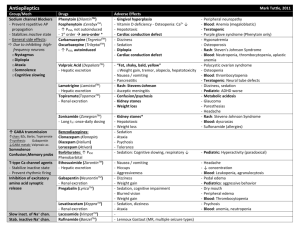
Supplementary Figure legends Supplementary Figure 1. Effect of the combinational therapy of nutlin-3 and valproic acid on viability of the wt p53 AML cell line MOLM-13. (A) The AML cell line MOLM13 (wild type TP53) was treated with nutlin-3 (0.5 - 10 M) and valproic acid (50 - 1000 M) alone or in combination for 24, 48 and 72 h (nutlin-3 added for the final 24 h). Cell viability was detected by DNA specific staining with Hoechst 33342. Viability data were analyzed in triplicate and data represents the results of three independent experiments (mean ± standard deviation). (B) Synergism for the interaction of nutlin-3 (5 M) and valproic acid (500 M) analyzed by Hoechst 33342 was calculated using Bliss independence, in which the fractional response of a combination of two drugs equals the sum of the two fractional responses minus their product. From the response to each of the drugs alone, the expected response to the combination at similar concentrations was calculated. A positive difference between actual response and expected response was then ascribed to synergy (*** p < 0.001). Error bars represent standard error of mean. (C) Representative fluorescence microscopy images of MOLM-13 and peripheral blood lymphocytes stained with DNA specific Hoechst 33342, following treatment with valproic acid (500 µM), nutlin-3 (5 µM), both or control (DMSO) for 48 h. White arrows represent apoptotic cells. Images are taken at 40x magnification. (D) MOLM-13 cells were treated with the combination of nutlin- (2.5 – 5 µM) and valproic acid (250 – 500 µM) and viability assessed by the colourimetric Alamar blue (24 h) and bioluminescence based ATP assays (48 h) (*p < 0.05, **0.01 or ***0.001). Synergism was calculated using Bliss Independence. Supplementary Figure 2. Effect of the combinational therapy of nutlin-3 and valproic acid on induction of autophagy. (A) MOLM-13 cells treated with valproic acid (500 M), nutlin-3 (5 M) or combination of both for 72 h (nutlin-3 for the last 24 h) were incubated 1 with Lysotracker Red for 30 minutes and analyzed by flow cytometry. Results are shown as median fluorescence intensity relative to control (*p < 0.05). (B) MOLM-13 cells were cells treated with valproic acid (500 M), nutlin-3 (5 M) or combination of both for 48 h (nutlin-3 for the last 24 h), and prepared for analysis by transmission electron microscopy (TEM) for detection of autophagic features. Arrows indicate autophagosomes. Supplementary Figure 3. Effect of the combinational therapy of nutlin-3 and valproic acid on viability of primary AML cells. (A) Example of AML patient sample treated with nutlin-3 (5 M), valproic acid (500 M) or combination of both for 48 h (nutlin-3 added for the final 24 h), followed by flow cytometric analysis of viability (Annexin-PI) and data analysis using FlowJo software version 8.2 (Tree Star, Inc., Ashland, OR, USA). (B) Differences in means of viability between nutlin-3 (5 M), valproic acid (500 M) or combination of both for 48 h (nutlin-3 added for the final 24 h) for the pooled patient data analyzed by Hoechst 33342. Results are given as means ± standard error of mean (* p < 0.05, n = 34). Supplementary Figure 4. Effect of the combinational therapy of nutlin-3 and valproic acid on viability of normal CD34+ cord blood compared to primary AML cells. CD34 positive cells were isolated from 3 different normal cord blood samples (positive selection), treated with nutlin-3 (5 M), valproic acid (500 M) or combination of both for 48 h (nutlin-3 added for the final 24 h) and flow cytometric analysis of viability was performed (AnnexinPI). The results were compared to the effect in the 10 most sensitive AML patient samples and the whole cohort of AML patient samples (n = 31). Results are given as means ± standard error of mean (* p < 0.05, *** p < 0.001). 2 Supplementary Figure 5. Development of an optically imageable MOLM-13 AML xenograft model. (A) Survival curve of NOD-scid (n = 6), NOD-scid 2mnull (n = 6) and NOD-scid IL2rnull (n = 10) injected i.v with 10 million MOLM-13 or MOLM-13luc cells following 3, 2.5, or 1.5 Gy irradiation, respectively. While of NOD-scid and NOD-scid 2mnull mice showed variable disease latency with not all animals developing leukemia, recipient NOD-scid IL2rnull all developed leukemia with median survival of 27 ± 0.8 days. (B) MOLM-13luc AML development in NOD-scid IL2rnull mice could be monitored longitudinally with bioluminescence imaging. The figure illustrates results of representative in vivo bioluminescence imaging of dorsal and ventral aspects of an animal injected with 10 million MOLM-13luc cells. Images were acquired 10 minutes following injection of 150 mg/kg of D-luciferin i.p, and performed once per week for 3 weeks and on day 24 after cell injection. Color bars represent photon counts per raster scan point (1 mm2) per second. (C) Quantification of total photon counts per second, per whole-body, region of interest plotted versus time (n = 4 mice). Inset shows total photon counts per second for dorsal and ventral regions of interest. (D) Following necropsy, liver, spleen, lymph nodes, femur, lungs and GItract were resected out and imaged. All organs showed bioluminescent signals, which was particularly intense at the cecum-ileum junction (white arrow) and also in the appendix. (E) (Middle panel) Photo comparing appendix and upper part of the large intestine of healthy NOD-scid IL2rnull mouse and MOLM-13luc xenografted NOD-scid IL2rnull in moribund condition. Hematoxylin and eosin staining of representative histology sections of the colon and ileum confirmed MOLM-13 cell infiltration. Microscopy images are 10x and (inset) 40x magnification. 3