Name: Date: Period: Energy, Wavelength, and Frequency

Name: Date: Period:
Energy, Wavelength, and Frequency Calculations Practice
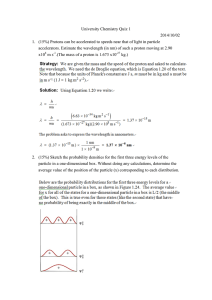
Hints: h= 6.63x10
-34 J·s & c=3.00x10
8 m/s
[Examples]
1.
The human eye can see light with a frequency about as high as 7.9x 10
14
Hz, which appears violet. Calculate the energy that one photon of violet light carries.
2.
Calculate the smallest increment of energy that is the quantum of energy, which an object can absorb from a red light whose wavelength is 772 m.
[We Do]
3.
What is the energy of a photon of light that has a frequency of 9.35 x10
21
Hz?
4.
An argon laser emits light at a wavelength of 4.89 x 10
-7 m. What is the energy of this light?
[You Do]
5.
Laser light is found in many types of technology including DVD players and surgical tools. If a laser emits light with a frequency of 5.0x10
14 Hz, what is the energy of this light?
6.
Ultraviolet (UV) radiation has enough energy, 6.626 x 10
-20
J, to penetrate the top layer of human skin, causing sunburn. What is the frequency of this radiation?
7.
How much energy carried by a photon of light that has a wavelength of 4.9x10
-7 m?
8.
What is the frequency of a photon of green light with an energy of 3.82 x 10-
19
J?
9.
When excited, some atoms produce an emission with a frequency of 7.25 x 10
12
Hz. Calculate the energy for one photon with this frequency.
10.
Calculate the energy of one photon of green light, which has a wavelength of 4.2 x 10 -9 m.