File
advertisement

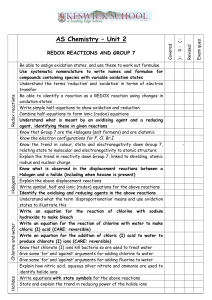
Electrochemistry Test Study Guide IN GENERAL metal ↔ metal ion+n + ne- (Example: Pb(s) ↔ Pb2+(aq) + 2e-) non-metal + ne- ↔ non-metal ion-n (Example: Cl2(g) + 2e- ↔ 2 Cl-(aq)) OXIDATION NUMBERS Rules for assigning oxidation numbers REDOX REACTIONS oxidizing agents vs. reducing agents reduction reactions vs. oxidation reactions be able to identify redox reactions (ie. use oxidation numbers) use change in oxidation numbers to identify the molecule/atom that is being oxidized, reduced, or is just a spectator ion DISPROPORTIONATION the same atom in the same molecule undergoes oxidation, while the same atom in another identical molecule undergoes reduction BALANCING REDOX REACTIONS always need the same amount of electrons being lost as there are electrons being gained!! 1. Half-Reactions Method o Use oxidation numbers to help identify which chemicals are being oxidized and which are being reduced o Use the 5 steps to balance each half reaction in acidic conditions o Balance the number of electrons in each half reaction (this will give the number of electrons transferred in the redox reaction) o Add the two half reactions together to get the balanced redox reaction 2. Oxidation Numbers Method o Use this method if asked to determine the number of electrons transferred per atom, per molecule, or per reaction SPONTANEITY RULE Spontaneous reactions occur if the oxidizing agent is higher than reducing agent on table on pg. 7 of data booklet Be able to build a reduction half-reaction table from a list of spontaneous and non-spontaneous reactions o Need to identify the OA and RA for each reaction and then rank the OA and RA based on the spontaneity rule REDOX STOICHIOMETRY Always need to begin with a balanced redox reaction. Several ways to develop a balanced redox reaction o Oxidation numbers method o Half-reactions method Half-reactions can be created by using the 5 steps if the reaction is in acidic conditions Half-reactions can be simple and can be created based on your knowledge of metals and non-metals Copy half-reactions from pg. 7 of data book for the strongest OA and for the strongest RA Stoichiometry calculations always involve 3 steps: 1. Calculate the moles of the chemical you have the most information for 2. Use your “want over have” ratio to calculate the moles of the chemical you are trying to find 3. Solve for the unknown Chapter Review Pg. 474-475 Reduction vs. Oxidation, RA vs. OA, and Redox Reactions in general: # 1, 2, 4, 5, 10 Oxidation Numbers: #3, 6 Disproportionation: #9 (omit 9a), 16a (just complete and balance the net ionic equation), Practice Problem Half-Reactions Method for Balancing: #14a-c, 17a-b Oxidation Numbers Method for Balancing: #15 Redox Stoichiometry: Practice Problem Practice Problem Highly toxic phosphine gas, PH3(g), is used in industry to produce flame retardants. One way to make phosphine on a large scale is by heating elemental phosphorus with a strong base as shown by the following reaction. P4(s) + 3H2O(l) + 3OH-(aq) 3H2PO2-(aq) + PH3(g) a. Show that the reaction is a disproportionation reaction. b. Calculate the mass of phosphine that can theoretically be made from 10.0kg of phosphorus by this method. [Answers: see #19b-c in text book]