Isotopes and Atomic Mass Lab, or Beanium Lab
advertisement

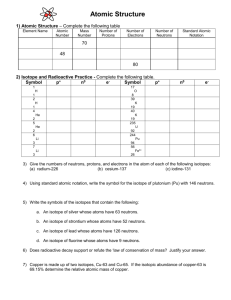
Isotopes and Atomic Mass Lab, or “Beanium” Lab Purpose: In this lab you will carry out experiments and perform the necessary calculations to determine the atomic mass of the fictitious element Beanium. These experiments and calculations are equivalent to the way scientists actually determine the atomic mass of elements. The three different isotopes of Beanium are beaniumblackium, beanium- brownium, and beanium-whitium. As in real elements, the mixture of isotopes are collections of atoms of the element each having different masses because they have different numbers of neutrons. Your job will be to obtain a sample of Beanium with all three of its representative isotopes, and determine the relative abundance of each isotope. From this data you will calculate the average mass of each isotope and the weighted average atomic mass of the element Beanium. Unlike real isotopes, the individual isotopic particles of Beanium differ slightly in mass, so you will determine the average mass of each type of isotopic particle. Then you can calculate the "weighted average atomic mass" of Beanium. Lab Report: You will complete the lab and begin to write your own lab report in class – one per student – NO GROUPS! Your lab report will have, labeled with roman numerals: I Introduction Section, II Materials Section, III Data Table Section, IV Calculations Section, V Conclusion section VI Questions You DO NOT need a Procedure Section in your lab report. Follow the procedure below: Before the Lab: 1) Write an Introduction Section, Materials Section and make a data table. In the introduction, describe in general what the lab is about and what you will do in general (not step by step). Be sure to include what isotopes are, and how the beans connect to actual isotopes. 2) Your data table which will have these headings across the top: Blackium, Brownium, Whitium, and Total. The rows will be labeled along the side as follows: Total Mass , Number, Average mass, and Percent abundance. 3) Then make a separate data table for step #6 of the procedure including rows for mass of largest, mass of the “average” size and mass of the smallest black bean. Procedure: 1. 2. 3. 4. 5. Obtain a sample of the Beanium “atoms” in a cup from the front of the room. Separate your beans by color. Count the number of each color of bean. Record on data table. Weigh all the black beans. Record mass on data table. Do the same with brown and white beans. Select three brown beans from your sample--the largest, the smallest, and one that appears to be "average" in size. Determine the mass of each of the three. 6. Place all the beans back in the container at the front of the room. Calculations: Calculate each of the following for each “Isotope” of the Element Beanium. SHOW WORK!! 1. Average Mass of one bean: Divide the total mass of the black beans by the total number of black beans. Record under “average mass” in data table. Do the same for the brown and white beans. Include calculations in the calculations section. Write results in data table. 2. Percent Abundance: Divide the number of each isotope (color bean) by the total number of particles (beans). Multiply this by 100 to get percent. Write results in the data table under percent abundance. 3. Average Atomic Mass: Use the percent abundances and the average masses in the atomic mass equation. Atomic mass = % of isotope #1 x (mass isotope #1) + % of isotope #2 x (mass Isotope #2) + of Beanium 100 100 % of isotope #3 x (mass Isotope #3) 100 Isotopes Discussion: (useful for your introduction – DO NOT COPY THIS) #Protons: determine which element an atom is – determines the atomic number # electrons: determine the charge on an atom Protons = electrons – atom is neutral Protons> electrons – atom is positively charged (lost electrons) Protons< electrons - atom is negatively charged (gained electrons) # neutrons – determines the atomic mass of an atom. Determines the isotope # of the atom. Isotope: an atom of an element with a certain number of neutrons. NOTE: All atoms of an element are isotopes of that element. Most elements have 1, 2 or 3 naturally occurring isotopes. This means that in any sample of the element these naturally occurring isotopes are all present typically always in the same % ratio. For example: The element carbon has three isotopes: 12 6 C = 6 neutrons and is called Carbon -12 isotope 90% abundance 13 6 C = 7 neutrons and is called carbon-13 isotope 9% abundance 14 6 C = 8 neutrons and is called carbon-14 isotope 1% abundance % abundance means that in a sample of carbon (like a lump of coal or a diamond) 90% of the carbon atoms will be carbon-12, 9% will be carbon-13 and 1% will be carbon-14. Since not all the atoms in a sample of an element have the same mass, we have to calculate an average atomic mass for the element. The average atomic mass is calculated taking into account the different percents of each isotope present. Avg. Atomic mass = % of isotope #1 x (mass isotope #1) +% of isotope #2 x (mass Isotope #2) + % of isotope #3 x (massIsotope #3) 100 100 100 In your introduction to the Beanium Lab you should include : What the purpose of the lab is What an isotope is How the three colors of beans represent isotopes How to calculate the atomic mass. Questions: Answer each question in complete sentences. Answer on a separate page or attach this page to your lab report. Your answers must be complete sentences and legible! Include any appropriate calculations! 1. 2. In the lab what represented the different isotopes of the element Beanium that would be like the isotopes of a real element? Explain how they represented isotopes. What was different about each “isotope” of Beanium besides color? What was the same? 3. Explain why there might be differences between the atomic mass of your Beanium sample and that of a different lab group. Explain why the difference would be smaller if larger samples were used for each group. 4. From the procedure you have data for 1) mass of one brown bean that is on the large side; 2) the mass of one brown bean that is on the small side; 3) the mass of one average size brown bean; and 4) the average mass of all the brown beans in your sample. Fill in the values below and answer the questions. Which do you think is the most accurate the average looking bean or the average of al brown beans? Why? Which is the least accurate? Why? Explain each answer!! Mass of small brown bean = Mass of average brown bean = Mass of large brown bean = Average mass of all brown beans = 5. The element Chlorine has two isotopes. Cl atomic mass 35 Percent Abundance = 76%; and Cl atomic mass 37, Percent Abundance 24%. What is the weighted average atomic mass of the element Chlorine? Compare your answer to the reported atomic mass for chlorine on your periodic table. SHOW CALCULATIONS!! Isotopes and Weighted Average Atomic Mass Name :___________________ Period: _______ If the atomic mass is the sum of protons and neutrons in an atom, why are the atomic masses on the periodic table not all whole numbers? Because the atoms of elements come in a variety of isotopes, meaning they are made up of atoms with the same number of protons (atomic number) but different number of neutrons in the nucleus. The atomic number on the periodic table is a “weighted average” of the naturally occurring isotopes of that element. The weighted average atomic mass (sum of neutrons and protons) takes into account the fact that there are different amounts of each type of isotope (abundance) in a naturally occurring sample of any given element. The weighted average atomic mass is calculated by multiplying the decimal equivalent of each isotope times its mass and adding up all the results for all the naturally occurring isotopes. AMU = atomic mass unit = mass of one proton = mass of one neutron Example: A sample of Cesium, Cs, has the following % abundance: Cs-132 = 20.0%; Cs – 133 = 75.3%; Cs – 134 = 4.7% Weighted average atomic mass = (0.753 x 133) + (0.20 x 132) + (134 x 0.047) = 132.85 amu ___________________________________________________________________ Determine the average atomic mass for the following mixtures of isotopes: 1.) Au – 197 = 50%, Au 198 = 50% 2.) Fe – 55 = 15%, Fe – 56 = 85% 3.) H – 1 = 99%, H-2- = 0.8%, H – 3 = 0.2% 4.) N-14 = 95%, N-15 = 3%, N-16 = 2% 5.) C-12 = 98%, C-14 = 2%