REVIEW FOR ATOMIC STRUCTURE EXAM State the charge for
advertisement
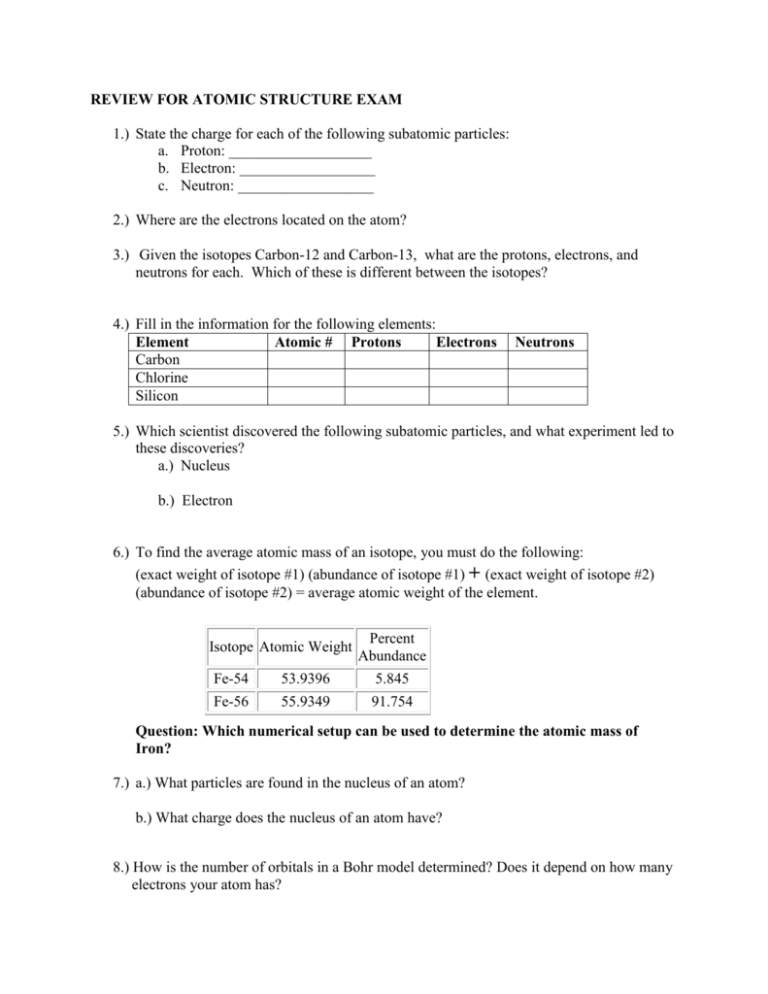
REVIEW FOR ATOMIC STRUCTURE EXAM 1.) State the charge for each of the following subatomic particles: a. Proton: ___________________ b. Electron: __________________ c. Neutron: __________________ 2.) Where are the electrons located on the atom? 3.) Given the isotopes Carbon-12 and Carbon-13, what are the protons, electrons, and neutrons for each. Which of these is different between the isotopes? 4.) Fill in the information for the following elements: Element Atomic # Protons Electrons Carbon Chlorine Silicon Neutrons 5.) Which scientist discovered the following subatomic particles, and what experiment led to these discoveries? a.) Nucleus b.) Electron 6.) To find the average atomic mass of an isotope, you must do the following: (exact weight of isotope #1) (abundance of isotope #1) + (exact weight of isotope #2) (abundance of isotope #2) = average atomic weight of the element. Isotope Atomic Weight Percent Abundance Fe-54 53.9396 5.845 Fe-56 55.9349 91.754 Question: Which numerical setup can be used to determine the atomic mass of Iron? 7.) a.) What particles are found in the nucleus of an atom? b.) What charge does the nucleus of an atom have? 8.) How is the number of orbitals in a Bohr model determined? Does it depend on how many electrons your atom has? 9.) Given element Neon and Calcium, Draw the Bhor models for each. Make sure you show the protons, electrons and neutrons as part of the Bhor model. 10.) Write down a summary of the gold foil experiment. Provide enough detail to explain who carried out the gold foil experiment, what part of the atom was discovered and explain why almost all alpha particles passed straight through the foil? 11.) Given Isotope: Boron-10. What does the 10 represent? What is the number of neutrons for this isotope? 12.) What is the nuclear symbol for Chlorine-35? What is the mass? 13) Which subatomic particle was discovered as a result of using a Cathode Ray Tube? 14) What happens to the wavelength and frequency as the energy of a photon increases?











