Eminemium Isotopes Lab: Atomic Mass Calculation
advertisement

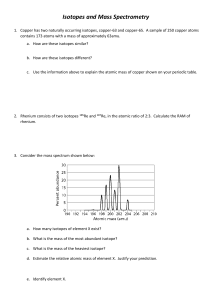
Lab: Isotopes of Eminemium PLEASE DO NOT WRITE ON THIS CLASS COPY Purpose: To determine the ATOMIC MASS of the element Eminemium (MM) by finding the MASS NUMBER of all the isotopes of Eminemium. Procedure: 1. Obtain a sample of Eminemium. 2. Create a data table in your notebook: Isotope Name Total Mass (g) # Atoms Avg Mass 1 Atom (g) Relative Abundance ALL Atoms 3. Zero the balance. Then zero it again with an empty cup on it. (Tare the balance). 4. Mass the whole sample and count how many atoms are in the sample. Record both in the “ALL Atoms” row of the data table. 5. Sort the original sample into the three isotopes – one per container. 6. Mass each isotope sample, and record in the “Total Mass” column of the data table for each sample. 7. Count the number of atoms in each isotope sample, and record in the “# Atoms” column of the data table. Analysis: 1. For each row, calculate and record the mass of one atom. 𝑇𝑜𝑡𝑎𝑙 𝑀𝑎𝑠𝑠 (𝑔) ) # 𝐴𝑡𝑜𝑚𝑠 ( = 𝑎𝑣𝑒𝑟𝑎𝑔𝑒 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 (𝑔) 2. For each isotope sample, calculate and record the relative abundance. # 𝐴𝑡𝑜𝑚𝑠 𝑜𝑓 𝐼𝑠𝑜𝑡𝑜𝑝𝑒 ( ) = 𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑇𝑂𝑇𝐴𝐿 # 𝐴𝑡𝑜𝑚𝑠 3. Go to the Chem Lab Methods website and click on the ENTER M&M DATA HERE link. Enter information requested by the form. 4. Extra challenge: Calculate ATOMIC MASS for Eminemium (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 1)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 1) + (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 2)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 2) + (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 3)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 3) = 𝐴𝑇𝑂𝑀𝐼𝐶 𝑀𝐴𝑆𝑆 𝑡𝑜𝑡𝑎𝑙 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐴𝐿𝐿 𝑎𝑡𝑜𝑚𝑠 ) # 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝐴𝐿𝐿 𝑎𝑡𝑜𝑚𝑠 Compare to: ( =? Clean-up: Evenly divide the Eminemium among all interested lab partners. Return cups to Ms. Schneider. Throw away garbage. Lab: Isotopes of Eminemium - Analysis: 1. For each row, calculate and record the mass of one atom. 𝑇𝑜𝑡𝑎𝑙 𝑀𝑎𝑠𝑠 (𝑔) ) # 𝐴𝑡𝑜𝑚𝑠 ( = 𝑎𝑣𝑒𝑟𝑎𝑔𝑒 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 (𝑔) 2. For each isotope sample, calculate and record the relative abundance. # 𝐴𝑡𝑜𝑚𝑠 𝑜𝑓 𝐼𝑠𝑜𝑡𝑜𝑝𝑒 ( ) = 𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑇𝑂𝑇𝐴𝐿 # 𝐴𝑡𝑜𝑚𝑠 3. Go to the Chem Lab Methods website and click on the ENTER M&M DATA HERE link. Enter information requested by the form. Extra challenge: Calculate ATOMIC MASS for Eminemium (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 1)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 1) + (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 2)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 2) + (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 3)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 3) = 𝐴𝑇𝑂𝑀𝐼𝐶 𝑀𝐴𝑆𝑆 𝑡𝑜𝑡𝑎𝑙 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐴𝐿𝐿 𝑎𝑡𝑜𝑚𝑠 ) # 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝐴𝐿𝐿 𝑎𝑡𝑜𝑚𝑠 Compare to: ( =? Lab: Isotopes of Eminemium - Analysis: 1. For each row, calculate and record the mass of one atom. 𝑇𝑜𝑡𝑎𝑙 𝑀𝑎𝑠𝑠 (𝑔) ) # 𝐴𝑡𝑜𝑚𝑠 ( = 𝑎𝑣𝑒𝑟𝑎𝑔𝑒 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 (𝑔) 2. For each isotope sample, calculate and record the relative abundance. # 𝐴𝑡𝑜𝑚𝑠 𝑜𝑓 𝐼𝑠𝑜𝑡𝑜𝑝𝑒 ( ) = 𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑇𝑂𝑇𝐴𝐿 # 𝐴𝑡𝑜𝑚𝑠 3. Go to the Chem Lab Methods website and click on the ENTER M&M DATA HERE link. Enter information requested by the form. Extra challenge: Calculate ATOMIC MASS for Eminemium (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 1)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 1) + (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 2)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 2) + (𝑟𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑎𝑏𝑢𝑛𝑑𝑎𝑛𝑐𝑒 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 3)(𝑎𝑣𝑔 𝑚𝑎𝑠𝑠 𝑜𝑛𝑒 𝑎𝑡𝑜𝑚 𝑜𝑓 𝑖𝑠𝑜𝑡𝑜𝑝𝑒 3) = 𝐴𝑇𝑂𝑀𝐼𝐶 𝑀𝐴𝑆𝑆 𝑡𝑜𝑡𝑎𝑙 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐴𝐿𝐿 𝑎𝑡𝑜𝑚𝑠 ) # 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝐴𝐿𝐿 𝑎𝑡𝑜𝑚𝑠 Compare to: ( =?