Pure Substances vs. Mixtures: Chemistry Lecture Notes
advertisement

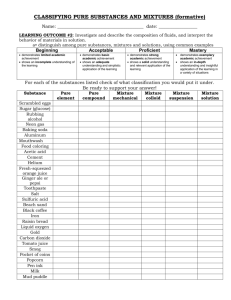
POM 11 Lecture Notes 1/1/2010 3:33:00 PM Purpose: Determine whether a substance is pure or a mixture; identify the criteria used to make that determination. Ask students to write their definitions of mixture and pure substance in their composition books. Have students complete questions 1-3 on student sheet 11.1 and then have students share out their ideas. Inform students: most raw materials are mixtures; for example crude oil; “pure” doesn’t mean “free of additives” or “safe to eat/drink” or “clean” Pure substance – matter that has definite chemical and physical properties. It can be an element or a compound that is combined chemically. A pure substance has constant composition and has consistent properties throughout the sample. Sodium chloride, water, diamonds…all good examples of pure substances. Compounds are pure substances. Mixture – matter that contains 2 or more substances that differ in physical and chemical properties AND are not combined chemically (no chemical reaction occurs between the substances). The substances keep their own physical properties (the sand is still sand and the water is still water). The substances are not present in a fixed ratio; for example it doesn’t matter if you add 20 g, 200 g, or 2000 g of sand to a bucket of water…it’s still a mixture of sand and water. A compound is not a mixture because: o The composition of a compound is constant throughout o Mixtures can be separated by physical means such as filtering; compounds are separated chemically. Types of Mixtures Homogeneous – the mixture is uniform in composition (appears to be one substance). A solution of salt water is an example. The salt dissolves and spreads evenly through the water in a way that makes it impossible to see the salt as separate from the water. Heterogeneous – not every part of the mixture is present in the same amount. The components posess clearly identifiable properties. Cereal in milk is a good example. You can still separate the parts out. Suspension – a mixture in which the particles of a material are dispersed throughout a liquid or gas but are large enough to settle out. Alloy – a homogeneous mixture of two or more elements, at least one of which is a metal, where the resulting material has metallic properties. Steel is an alloy made mainly of iron with a small amount of carbon (to make it harder), manganese (to make it stronger), and chromium (to make it resistant to rusting). Colloid – particles are dispersed but do not settle out. 1/1/2010 3:33:00 PM 1/1/2010 3:33:00 PM