Enthalpy
advertisement

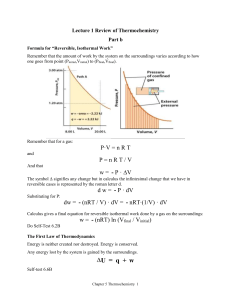
Formula for “Reversible, Isothermal Work” Remember that the amount of work by the system on the surroundings varies according to how one goes from point (Pinitial,Vinitial) to (Pfinal,Vfinal). Remember that for a gas: P·V = n R T and P=nRT/V And that w = - P · V The symbol signifies any change but in calculus the infintesimal change that we have in reversible cases is represented by the roman letter d. d w = - P · dV Substituting for P: d w = - (n R T / V) · dV Calculus gives a final equation for reversible isothermal work done by a gas on the surroundings: w = - (n R T) ln (Vfinal / Vinitial) Do Self-Test 6.2B The First Law of Thermodynamics Energy is neither created nor destroyed. Energy is conserved. Any energy lost by the system is gained by the surroundings. U = q + w Self-test 6.6B Chapter 5 Thermochemistry 1 State Functions A state function is a property of a system that can be determined by specifying its final and initial conditions (in terms of temperatue, pressure, etc). The value of a state function does not depend on the particular history of the sample, only its present condition. The change in the state function depends only on the initial and final states of the system, not how the change occurs. Can use an “energy diagram”. Work, w and heat, q are not state functions because they depend on the path taken Heat at constant pressure Consider the case where no non-expansion work is done and where the volume of the system does not change. Since V=0, w=0 and all the heat q involved in the change is thus equal to the internal energy. U = q + w = qv (const vol, w=0) This means that when a chemical reaction is performed in a constant volume calorimeter (a “bomb”calorimeter), the heat measured is the internal energy, U. Because the heat capacity, C equals q/T, CV = U /T Chapter 5 Thermochemistry 2 Heat at constant pressure Enthalpy Heat transferred under constant-pressure conditions is called enthalpy, H. It is related to the internal energy by H = U + PV Consider the case for a change when all the work done by the system on the surroundings is expansion under constant pressure. H = U + PV but the internal energy equals the heat and work: U = qp - PexV H = (qp - PexV) + PV The change in enthalpy, ∆H, equals the heat, qp, gained or lost by the system when the process occurs under constant pressure: H H final Hinitial q p If ∆H is positive then the system has gained heat from surroundings, and is an endothermic process. If ∆H is negative, the system has released heat to the surroundings, and is an exothermic process. Enthalpy, H, is a state function; heat in general, q, is not. Since heat transferred at constant pressure is identified as the change in enthalpy, ∆H, we can define the heat capacity at constant pressure, CP as Chapter 5 Thermochemistry 3 CP = H /T The heat involved in a reaction at constant pressure can be measured with a simple apparatus called the “coffee cup” calorimeter: [Section 6.10 of textbook will not be covered in this course.] Enthalpies of Physical Change The molecules in a solid are vibrating in place. Temperature is a measure of the average kinetic energy that the molecules. As the temperature rises, more kinetic energy is added and the molecules vibrate more. At one particular temperature, the molecules begin to tumble past each other. This breaking loose to tumble around each other (still in contact with each other) requires more energy. This is called the latent energy. At constant pressure, the molar enthalpy change that accompanies melting (fusion) is called the enthalpy of fusion, ∆Hfus. ∆Hfus = H (liquid) – H (solid) Chapter 5 Thermochemistry 4 Fusion is the “act of making fluid” and is the opposite of freezing. The enthalpy of freezing is equal and opposite to ∆Hfus. The amount of energy needed to vaporize a liquid into a gas is much more than that needed to melt (fuse). This is because the molecule in a gas are no longer in contact. The difference in molar enthalpy between the vapor and liquid states of a substance is called the enthalpy of vaporization, ∆Hvap = H(gas) – H (liquid). Sublimation is the direct conversion of a solid into a gas. Frost disappearing is an example, as is the disappearance of “dry ice”, CO2(s). The enthalpy of sublimation, ∆Hsub = H(gas) – H (solid) = ∆Hfus + ∆Hvap Self-test 6.10B Chapter 5 Thermochemistry 5 Heating Curves Chapter 5 Thermochemistry 6 Enthalpies of Reaction H Hproducts Hreactants heat of reaction, ∆Hrxn 2H2 g O2 g 2H2Og H 483.6 kJ enthalpy – viewed as a measure of how much heat stored as potential energy in the system or as ‘heat content.’ Guidelines 1. Enthalpy is an extensive property. Magnitude of ∆H is directly proportional to amount of reactant consumed in process. CH4 g 2O2 g CO2 g 2H2Ol H 890 kJ The combustion of 2 mol of CH4 would release –1780 kJ. 2 (CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H = +890 kJ ) 2CH4(g) + 4O2(g) 2CO2(g) + 4H2O(l) H = +1780 kJ 2. The enthalpy change for a reaction is equal in magnitude but opposite in sign to ∆H for reverse reaction. Chapter 5 Thermochemistry 7 CO2 g 2H2 Ol CH4 g 2O2 g H 890 kJ 3. The enthalpy change for a reaction depends on the state of the reactants and products. 2H2O(g) 2H2O(l) H = - 88 kJ So adding the two equations ( the H2O(l) on both sides cancel out): CO2(g) + 2H2O(l) CH4(g) + 2O2(g) H = +890 kJ 2H2O(g) 2H2O(l) H = - 88 kJ --------------------------------------------------------------------CO2(g) + 2H2O(g) CH4(g) + 2O2(g) H = +802 kJ 4. These are thermochemical equations and the H quantity is stoichiomentric Example: When one mole of ethylene, C2H6(g) is combusted to CO2(g) and H2O(g), 316.3 kcal of heat are liberated under constant pressure. If 78.6g CO2(g) are produced from this reaction, calculate the enthalpy change for this reaction. Chapter 5 Thermochemistry 8 Step 1: Balance the thermochemical equation C2H6(g) + 3O2(g) 2CO2(g) + 2H2O(g) H = -316.3 kcal Step 2: Calculate the molar relation 78.6g CO2 · 1 mol CO2 · -316.3 kcal 44.0 g CO2 2 mol CO2 = - 283. kcal Self-test 6.11B Hess’s Law Hess’s law states that if a reaction is carried out in a series of steps, ∆H for the reaction will be equal to the sum of the enthalpy changes for the individual steps. Hess pointed out that the heat absorbed (or evolved) in a given chemical reaction is the same whether the process takes one step or in several steps. CO(g) + 1/2 O2 2 C(graph) + O2(g) H2 H3 H1 1 Chapter 5 Thermochemistry 9 3 CO2 We can burn graphite with excess O2 to get CO2 and measure the heat evolved, but it is technically very difficult to burn graphite and get only CO. We take advantage of the fact that CO burns to give CO2. H2 = H1 - H3 H1 C + O2 CO2 CO2 CO + ½ O2 -H3 --------------------------------H2 C + ½ O2 CO Tool Box 6.1 Do Self-tests 6.13,14B Enthalpies of Formation Enthalpy of formation, ∆Hf,(or heat of formation), the formation of a compound from its constituent elements. The standard enthalpy of formation of a compound, H f , is the change in enthalpy for the reaction that forms 1 mol of the compound from its elements, with all substances in their standard states. The most stable form of the element is used. E.g. O2 not O3, C(graphite not diamond) The standard enthalpy of formation of the most stable form of any element is zero. Using Enthalpies of Formation to Calculate Enthalpies of Reaction We can use Hess's law to calculate the standard enthalpy change for any reaction for which we know the H f values for all reactants and products. Chapter 5 Thermochemistry 10 5 ↓ (missing from eqn) example: C3 H8 g O2 g 3CO2 g 4 H2 Ol Hrxn nH f products mH f reactants From Appendix 2: H f Molecule (kJ/mol) C3H8(g) -2220. O2(g) 0 CO2(g) -393.51 H2O(g) -241.82 H2O(l) -285.83 (not all tables give values for elements in most stable form) Horxn = [3mol(-393.51kJ/mol) + 4mol(-285.83kJ/mol)] – [1mol(-2220.kJ/mol) + 5mol(0kJ/mol)] = - 104. kJ Self Tests 6.15,16B Section C of notes will include 6.19,20,21 Chapter 5 Thermochemistry 11