Week 1
advertisement

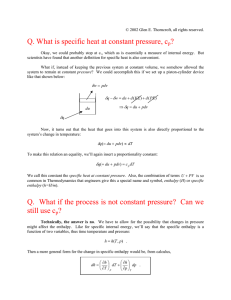
Thermodynamics Week 1 First Law Conservation of Energy: "There is a state function E, called ‘energy’, whose differential equals the work exchanged with the surroundings during an adiabatic process." Clausius Most often expressed as: where U is the internal energy of a system, Q is heat, and W is work. System An ensemble of bodies that is set aside for study. Important systems include: Isolated system – No matter or energy is exchanged with the surroundings. Closed system – No matter exchanged between the system and the surroundings. Open system – Matter and energy can be exchanged with the surroundings. State A system can be defined by state functions. The variables in state functions include volume (V), temperature (T), pressure (p) and concentration (ci) Internal energy is a state function and does not depend on path taken. Work Work is made up of mechanical work (p.dV) and non-mechanical work (electrical, magnetic etc.). Both heat and work are not state functions and depend upon the path taken. Extensive and Intensive Properties Extensive properties depend upon the extent of mass in a system. Intensive properties do not depend upon the extent of mass in a system. Intensive thermodynamic properties are most often prefixed with specific. In Ragone an underscore is used to denote properties per unit mass (U, V, etc). Specific volume V is the reciprocal of density ρ. Open System In an open system, matter may enter or leave the system. When writing the first law energy balance we must account for the internal energy of the matter leaving or entering the system. We must also account for the work done on the system when matter leaves or enters. This work is often called ‘flow work’. But Vi=Vimi and dVi=dVimi For a system with matter moving in (i) and out (o) can therefore write: More generally for multiple streams of matter in and out of the system Enthalpy The term U+PV is defined as enthalpy. It is designated by the term H. Enthalpy is an extensive property and Specific Enthalpy is given by: H=U+PV Heat Capacity at Constant Volume The amount of thermal energy required to change the temperature of a material is the heat capacity of the material. For a closed system at constant volume (dV=0) this can be written as: We can therefore define the heat capacity at constant volume as: Heat Capacity at Constant Pressure A material heated at constant pressure usually expands (i.e. it does work). At constant pressure dP=0 and d(PV)=VdP+PdV=PdV Example 1 1g of ice at T=0° C and 10g of water at 100° C are mixed in an adiabatic container. What is the temperature in the container when its contents reach equilibrium? Express your answer in K. For H2O the change in enthalpy on melting =6.01 kJ/mol and Cp=75.3 J/K. Three processes take place: (1) the ice melts – with an associated cost in energy; (2) the water produced from the ice warms up to the equilibrium temperature; (3) the 100° C water cools down to the equilibrium temperature. The change in enthalpy of the system is 0 therefore: