Phase Diagram Review - Liberty Union High School District
advertisement
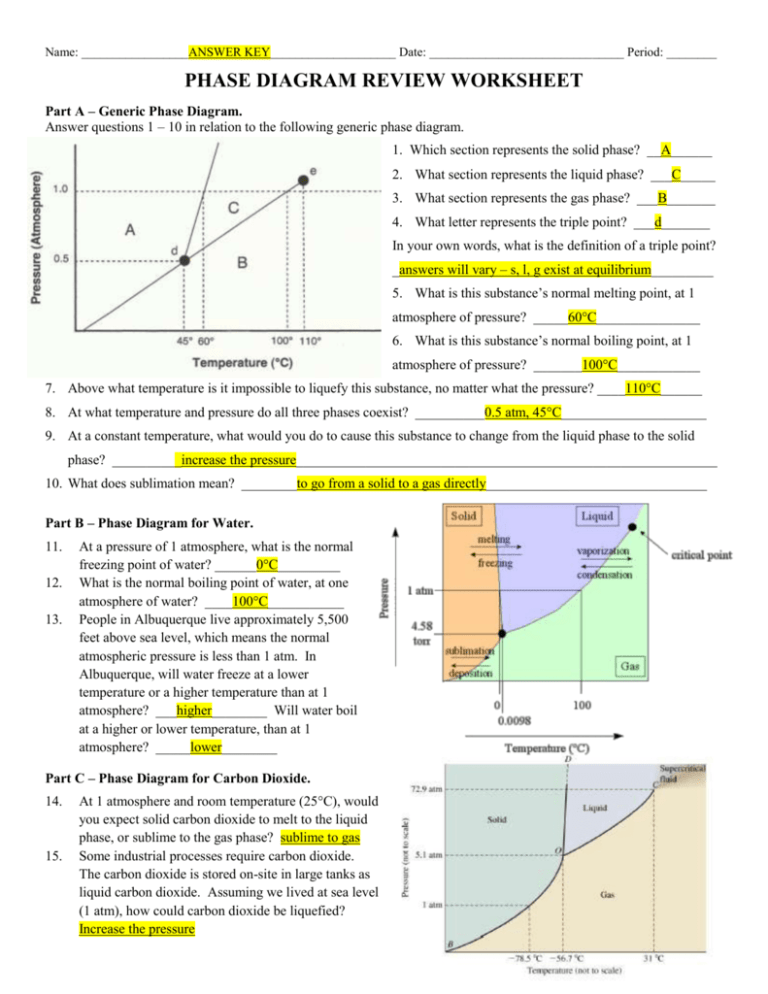
Name: _________________ANSWER KEY____________________ Date: _______________________________ Period: ________ PHASE DIAGRAM REVIEW WORKSHEET Part A – Generic Phase Diagram. Answer questions 1 – 10 in relation to the following generic phase diagram. 1. Which section represents the solid phase? __A______ 2. What section represents the liquid phase? ___C_____ 3. What section represents the gas phase? ___B_______ 4. What letter represents the triple point? ___d_______ In your own words, what is the definition of a triple point? _answers will vary – s, l, g exist at equilibrium_________ 5. What is this substance’s normal melting point, at 1 atmosphere of pressure? _____60°C_______________ 6. What is this substance’s normal boiling point, at 1 atmosphere of pressure? _______100°C____________ 7. Above what temperature is it impossible to liquefy this substance, no matter what the pressure? ____110°C______ 8. At what temperature and pressure do all three phases coexist? __________0.5 atm, 45°C_____________________ 9. At a constant temperature, what would you do to cause this substance to change from the liquid phase to the solid phase? __________increase the pressure_____________________________________________________________ 10. What does sublimation mean? ________to go from a solid to a gas directly________________________________ Part B – Phase Diagram for Water. 11. 12. 13. At a pressure of 1 atmosphere, what is the normal freezing point of water? ______0°C_________ What is the normal boiling point of water, at one atmosphere of water? ____100°C___________ People in Albuquerque live approximately 5,500 feet above sea level, which means the normal atmospheric pressure is less than 1 atm. In Albuquerque, will water freeze at a lower temperature or a higher temperature than at 1 atmosphere? ___higher________ Will water boil at a higher or lower temperature, than at 1 atmosphere? _____lower________ Part C – Phase Diagram for Carbon Dioxide. 14. 15. At 1 atmosphere and room temperature (25C), would you expect solid carbon dioxide to melt to the liquid phase, or sublime to the gas phase? sublime to gas Some industrial processes require carbon dioxide. The carbon dioxide is stored on-site in large tanks as liquid carbon dioxide. Assuming we lived at sea level (1 atm), how could carbon dioxide be liquefied? Increase the pressure Part D – Phase Diagram for Tastegudum On Crosbia, bolonium (Bg) and manasium (Ma) react together to form the compound tastegudum. For each of the following questions (16-28), refer to the phase diagram for tastegudum. See Miss Scott for answer key with labels 16. Label the regions of the diagram that correspond to the solid, liquid, and vapor phases. (Write the names of these phases in the appropriate regions directly on the diagram.) 17. Draw a small red circle around the point that is the critical point for tastegudum. 18. Draw a small blue circle around the point that is the triple point for tastegudum. 19. What is the critical pressure, Tp, of tastegudum? ______90 atm_____________ 20. What is the critical temperature, Tc, of tastegudum? ____750°C_____________ 21. At what temperature and pressure will all three phases of tastegudum coexist at equilibrium? T = ___250°C_____ P = ___35 atm_____ 22. What is the boiling point temperature for tastegudum when the external pressure is 60 atmospheres? ___500°C__ 23. What is the freezing point temperature for tastegudum when the external pressure is 60 atmospheres? __300°C___ 24. If you were to have a container containing tastegudum in your kitchen, in what state (phase of matter) would you expect to see it? Explain your answer. Gas – because we live at 1.0 atm of pressure and room temperature is 25°C 25. A container of tastegudum is sitting at a pressure of 45 atmospheres and temperature of 100 °C. Describe what will happen as the temperature is raised by 400 °C. It will go through sublimation (solid to gas) 26. A container of tastegudum is sitting at a temperature of 300 °C under 1 atmosphere of pressure. Describe what will happen as the pressure is increased to 90 atmospheres. It will go from gas to solid (deposition) 27. Why can tastegudum not be brought to a boil at a temperature of 200 °C? Cannot exist as a liquid at that temperature and boiling is liquid to gas. 28. Assuming it is not poisonous; could tastegudum be used as a drink on earth? Explain your answer. No, answers with vary.