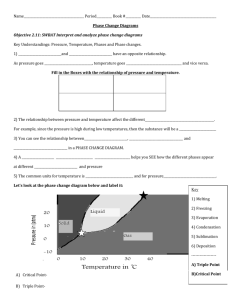
Phase Diagrams Any temperature and pressure combination
advertisement

Phase Diagrams • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. • Given a temperature and pressure, phase diagrams tell us which phase will exist. • Features of a phase diagram: – Triple point: temperature and pressure at which all three phases are in equilibrium. – Vapor-pressure curve: generally as pressure increases, temperature increases. – Critical point: critical temperature and pressure for the gas. – Melting point curve: as pressure increases, the solid phase is favored if the solid is more dense than the liquid. – Normal melting point: melting point at 1 atm. video Phase Diagrams • Any temperature and pressure combination not on a curve represents a single phase. Phase Diagrams The Phase Diagrams of H2O and CO2 • Water: – – – – – The melting point curve slopes to the left because ice is less dense than water. Triple point occurs at 0.0098C and 4.58 mmHg. Normal melting (freezing) point is 0C. Normal boiling point is 100C. Critical point is 374C and 218 atm. • Carbon Dioxide: – Triple point occurs at -56.4C and 5.11 atm. – Normal sublimation point is -78.5C. (At 1 atm CO2 sublimes it does not melt.) – Critical point occurs at 31.1C and 73 atm. Phase Diagrams The Phase Diagrams of H2O and CO2 • Critical Point: indicates the critical temperature and critical pressure • Critical Temperature: temperature above which the substance cannot exist in the liquid state • Critical Pressure: lowest pressure at which the substance can exist as a liquid at the critical temperature Triple Point of Water Substance T (K) P (kPa*) Acetylene 192.4 120 Ammonia 195.40 6.076 Argon 83.81 68.9 Butane[5] 134.6 7 × 10−4 Carbon (graphite) 4765 10132 Carbon dioxide 216.55 517 Carbon monoxide 68.10 15.37 Chloroform[6] 175.43 0.870 Deuterium 18.63 17.1 Ethane 89.89 8 × 10−4 Ethanol[7] 150 4.3 × 10−7 Ethylene 104.0 0.12 Formic acid[8] 281.40 2.2 • At the critical point C, the properties of the gas and liquid phases approach one another, resulting in only one phase: a homogeneous supercritical fluid It can diffuse through solids like a gas, and dissolve materials like a liquid Supercritical CO2 Used to Decaffeinate Coffee is any substance at a temperature and pressure above its critical point Copyright 1999, PRENTICE HALL Chapter 11 8 Applications • • • • Decaffeinating coffee Solvents for dry-cleaning Refrigeration Biodiesel production • Ice melts at a higher temperature with decreasing pressure Application • Freeze drying food: freeze food and then lower pressure so ice sublimes