Chapter 6 - Hope Charter School
advertisement

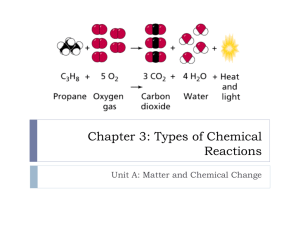
Honors Chemistry Chapter 6 notes—Chemical Equations and Reactions I. Chemical Equations A. Chemical Reactions 1. When two chemicals react the resulting compound(s) have different properties than the originals 2. There are signs that a reaction has occurred, but these alone do not prove it. a. color change b. precipitation of a solid c. heat, sound, or light are produced or absorbed d. odor changes e. gas is released B. Chemical Equations 1. Introduction a. Reactant—substance that undergoes a reaction b. Product—substance that is formed from a reaction c. word descriptions are not sufficient to describe the reaction 2. Balanced equations a. give us the reactants and the products b. what we start with, we must end with (law of mass conservation p. 40) c. can be done with words; however d. chemical equations are better e. physical state is frequently indicated 1) aq—aqueous, dissolved in water 2) s—solid 3) l—liquid 4) g—gas 5) ↑--gas produced 6) ↓--precipitate formed f. Energy and equations 1) Endothermic—reaction absorbs energy 2) Exothermic—reaction gives off energy 3) the word energy is only written in the equation if it is important to know whether energy is released or absorbed 3. Writing Balanced equations a. Word Form b. Symbol Form 1) write symbols 2) balance formulas with subscripts c. Balanced equation—use coefficients II. Types of Reactions A. Why classified? 1. Easier to remember familiar reactions 2. Make predictions about unfamiliar reactions B. Major classes of reactions 1. Synthesis reactions a. Two or more compounds combining to form ONE compound b. General equation: X + Y XY c. 4Fe(s) + 3O2 2Fe2O3(s) 2. Decomposition reactions a. One compound breaks down into two or more substances b. General equation: XY X + Y c. 2HgO 2Hg + O2 3. Single-displacement (single-replacement) reaction a. One element takes the place of another b. General equations: 1) XY + Z ZY + X 2) XY + W XW + Y c. Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s) d. Cl2(g) + 2NaBr(aq) 2NaCl(aq) + Br2(l) e. Reactivity series—standard reduction potentials 4. Double-displacement (double-replacement) reaction a. positive ions of two ionic compounds are interchanged b. General equation: XY + ZW ZY + XW c. Pb(NO3)2(aq) + 2KCl(aq) PbCI2(s) + 2KNO3(aq) d. one product must be water or a precipitate 5. Combustion reactions a. a substance reacts rapidly with oxygen to form an oxide b. General equation: X + O2 XO c. C + O2 CO2 III. Nature of Reactions A. Reversible reactions 1. Reactions that can go either way are reversible 2. Some cannot go either way and are said to go to completion a. at least one reactant is completely used up b. food is digested, paint hardens, fuel burns 3. Equilibrium a. when there is no net change occurring in the amount of reactants and products b. Dynamic equilibrium when both directions are occurring at the same time c. subway analogy d. Double arrow is used to indicate the equation is reversible (↔, half arrows) e. CaCO3(s) ↔ CaO(s) + CO2(g) f. Amounts of reactants and products are not necessarily equal. The reaction will favor the more stable compounds g. Le Chateliers principle—if stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. 1) If we remove product, then the reaction will continue to reach equilibrium CaCO3(s) CaO(s) + CO2(g) 2) If we add product (energy for instance) the reaction will move towards the right 3H2(g) + N2(g) 2NH3(g) + energy h. Solubility—the ability to be dissolved 1) Soluble—a compound is able to dissolve 2) Insoluble—a compound is not able to dissolve a) precipitate—solid separating from a solution, limits is ability to react with substances in solution, thereby causing the reaction to move right i. Effects of energy 1) Adding energy to an endothermic reaction pushes it right. 3C + 2Al2O3(s) + energy 4 Al(l) + 3 CO2(g) 2) Adding energy to an exothermic reaction pushes it left. 3H2(g) + N2(g) 2NH3(g) + energy B. Activation Energy 1. The energy necessary for a reaction to take place. 2. High activation energies indicates the reaction will proceed slowly C. Rate of Reaction 1. Effect of temperature a. raising the temperature generally makes reactions to go faster 2. Effect of concentration a. raising the concentration of a reactant will speed of the reaction b. lowering the concentration of a reactant will decrease the speed of the reaction c. limiting reactant—the one that there isn’t enough to keep the reaction going D. Catalysts 1. Defined—a substance that increase the rate of a reaction but does not enter into that reaction 2. Lowers the activation energy 3. Enzymes—organic catalyst 4. Inhibitor—substance that decreases the rate of a reaction but do not enter into the reaction