Approach to Acid Base
advertisement

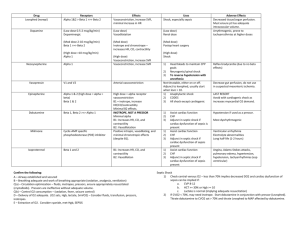
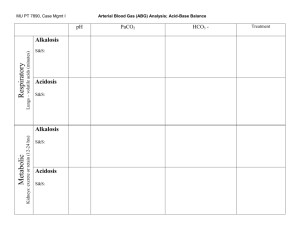
Simple Approach to Acid-Base Disorders 5 Steps to evaluation of an acid-base disturbance Before you begin, you must have an ABG and Chem-7 drawn at the same time. The HCO3 “bicarb” in these calculations comes from the Chem-7, NOT the ABG. Here are the 5 steps described below: 1. Look at the pH to determine if the patient has an acid-emia or alkal-emia. 2. Determine the primary type of disturbance. 3. Evaluate for compensation for the primary disturbance If there is an acidosis… 4. Determine if there is an anion gap. 5. If an elevated anion gap metabolic acidosis is present, check for the delta-delta. 1. Look at the pH to determine if the patient has an acid-emia or alkal-emia. This is different from an acidosis or alkalosis, which refers to the disorders. Determining if the patient has an alkalemia or academia is simple. If the pH is >7.42, they are alkalemic and the primary process is an alkalosis. If the pH is <7.38, the have an academia and the primary process is an acidosis. 2. After determining acidemia or alkalemia, determine the primary type of disturbance. Type of Disorder pH Metabolic Acidosis Decreased Metabolic Alkalosis Increased Respiratory Acidosis Decreased Respiratory Alkalosis Increased *Change is due to compensation PaCO2 Decreased* Increased* Increased Decreased HCO3 Decreased Increased Increased* Decreased* 3. Evaluate compensation for the primary disturbance Disorder Metabolic Acidosis Primary Change Decrease in HCO3 Metabolic Alkalosis Increase in HCO3 Acute Respiratory Acidosis Increase in PaCO2 Chronic Respiratory Acidosis Increase in PaCO2 Compensation Winter’s equation: PaCO2=1.5[HCO3] + 8 -ORFor every decrease of HCO3 by 10, PaCO2 decreases by 12. For every increase in HCO3 by 10, the PaCO2 increases by 6. -ORIncr PaCO2 = 0.75 x change in HCO3 For every increase in PaCO2 by 10, the HCO3 increases by 1 -ORIncr HCO3 = 0.1 x change in PaCO2 For every increase in PaCO2 by 10, the HCO3 increases by 4. -ORIncr HCO3 = 0.4 x change in PaCO2 Acute Respiratory Alkalosis Decrease in PaCO2 Chronic Respiratory Alkalosis Decrease in PaCO2 For every decrease in PaCO2 by 10, the HCO3 decreases by 2. -ORDecr HCO3 = 0.2 x change in PaCO2 For every decrease in PaCO2 by 10, the HCO3 decreases by 5. -ORDecr HCO3 = 0.4 x change in PaCO2 Remember, appropriate compensation is NOT a secondary acidosis or alkalosis. But, too much or not enough compensation does indicate a second disorder. 4. Calculate the anion gap. This helps differentiate between AG met acidosis and non-AG met acidosis. Always calculate the AG. This may expose a hidden metabolic disturbance. AG = Na – (Cl + HCO3) Normal AG is around 12. 5. If an elevated anion gap metabolic acidosis is present, check for the “deltadelta” ratio. This is found only in patients with an AG met acidosis. What this rule says is that for every increase in AG, there should an equal decrease in the serum bicarbonate level. For example, if the AG is 20 (8 above normal), the expected HCO3 should be 16, (8 below a normal value of 24). If the expected bicarb is higher than 16, there is too much bicarb and another disturbance that is a metabolic alkalosis is present. If it is less than 16, there is another disturbance that is a NON-AG metabolic acidosis. Brief Diffential List: AG Met Acidosis – Methanol, Urea, DKA, Paraldahyde, Inorganic Phosphates, Lactic Acidosis, Ethylene Glycol/Ethyl Alcohol, Salicylates. Non-AG Met Acidosis – Diarrhea, Ureteroenteric Fistual, RTA, Hyperalimentation, Acetazolamine/Addison’s, Miscellaneous (toluene glue sniffing) Resp. Alkalosis – Hypoxia, sepsis, liver disease, salicylates, pain. Resp. Acidosis – Lung disease, CNS hypoventilation, sedatives, neuromuscular weakness Met Alkalosis – Chloride responsive (Cl is low) – diuretics, hypovolemia, vomiting, NG suction. Chloride unresponsive (Cl is nml or high) – renal decreased HCO3 excretion or endocrine – mineralcorticoid excess. Additonal Pearls: Anion Gap = unmeasured anions in blood. Albumin, phosphate, and sulfate are unmeasured anions. If albumin is low, then the “normal range” of AG should be lower. o Thus, for every decrease in albumin of 1, the AG should decrease 2.5 Salicylates cause an AG acidosis + a respiratory alkalosis Ketones for ketoacidosis – alcohol, diabetic, starvation Tox screen Lactate for lactic acisosis – hypoperfusion, ischemia, sepsis Osmolal gap – for other “unmeasured anions” like ethylene glycol Urine anion gap – for workup of NAG acidosis Sample Questions 1. 25 y.o. AAF with history of DM I presents with nausea, vomiting and polyuria. Na 128 K 6.5 Cl 90 Bicarb 10 BUN 40 Cr 2.0 Gluc 500 Ca 8.2 Mg 1.8 Phos 2.5 ABG – pH 7.00, pCO2 25, pO2 80, Bicarb 8, O2 Sat 98% on RA 2. 72 y.o. WM with 50 pack year history of tobacco use presents with AMS and shortness of breath. Na 145 K 4.5 Cl 100 Bicarb 35 BUN 15 Cr 1.0 Gluc 100 ABG – pH 7.20, pCO2 90, pO2 60, Bicarb 33, O2 Sat 88% on RA 3. 88 y.o. WF with dementia and recent antibiotic treatment for bronchitis comes in with profuse bloody diarrhea. Na 130 K 4.0 Cl 105 Bicarb 15 BUN 50 Cr 2.0 Gluc 100 ABG – pH 7.20, pCO2 30, pO2 80, Bicarb 13, O2 Sat 98% on RA 4. 24 y.o. WF with asthma presents with acute onset wheezing and shortness of breath not relieved by albuterol prn. Vitals – Temp 98.6, HR 110, RR 34, BP 100/60 ABG – pH 7.48, pCO2 20, pO2 80, Bicarb 20, O2 Sat 92% on RA Treatment? Despite therapy she continues to worsen, you know to recheck vitals and an ABG. Vitals – Temp 98.6, HR 110, RR 20, BP 100/60 ABG – pH 7.40, pCO2 40, pO2 80, Bicarb 24, O2 Sat 92% on RA What do you think is going on? Treatment? 5. After eating at Golden Corral a 30 y.o. WM develops acute onset nausea and vomiting 2 hours later. Vitals – Temp 99.6, HR 110, RR 14, BP 100/60 Na 145 K 4.5 Cl 90 Bicarb 44 BUN 10 Cr 1.0 Gluc 100 ABG – pH 7.50, pCO2 52, pO2 80, Bicarb 40, O2 Sat 98% on RA 6. A patient with long standing alcohol use and cirrhosis presents with altered mental status. Pt disheveled, breath smells like alcohol. Na 130, K 3.2, Cl 100, HCO3 20, BUN 18, Cr 1.1, Glu 60 INR 2.1, Albumin 2.0. ABG – 7.26/30/85 What is the corrected AG? What is going on? Work up? Treatment? JCH/acc 6/3/09