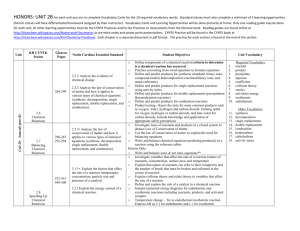
HONORS: UNIT 2B: Antacids Below are the class objectives
advertisement
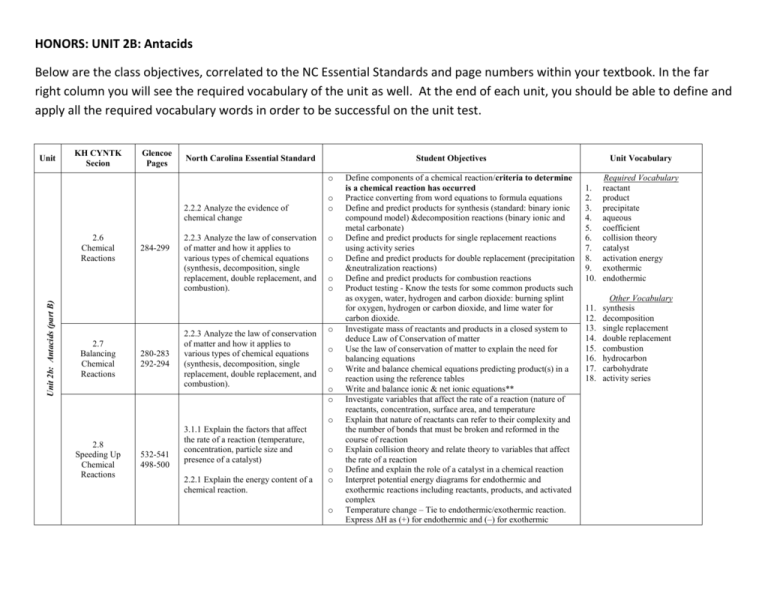
HONORS: UNIT 2B: Antacids Below are the class objectives, correlated to the NC Essential Standards and page numbers within your textbook. In the far right column you will see the required vocabulary of the unit as well. At the end of each unit, you should be able to define and apply all the required vocabulary words in order to be successful on the unit test. Unit KH CYNTK Secion Glencoe Pages North Carolina Essential Standard Student Objectives o 2.2.2 Analyze the evidence of chemical change Unit 2b: Antacids (part B) 2.6 Chemical Reactions 2.7 Balancing Chemical Reactions 284-299 280-283 292-294 2.2.3 Analyze the law of conservation of matter and how it applies to various types of chemical equations (synthesis, decomposition, single replacement, double replacement, and combustion). 2.2.3 Analyze the law of conservation of matter and how it applies to various types of chemical equations (synthesis, decomposition, single replacement, double replacement, and combustion). o o o o o o o o o o o o 2.8 Speeding Up Chemical Reactions 532-541 498-500 3.1.1 Explain the factors that affect the rate of a reaction (temperature, concentration, particle size and presence of a catalyst) 2.2.1 Explain the energy content of a chemical reaction. o o o o Define components of a chemical reaction/criteria to determine is a chemical reaction has occurred Practice converting from word equations to formula equations Define and predict products for synthesis (standard: binary ionic compound model) &decomposition reactions (binary ionic and metal carbonate) Define and predict products for single replacement reactions using activity series Define and predict products for double replacement (precipitation &neutralization reactions) Define and predict products for combustion reactions Product testing - Know the tests for some common products such as oxygen, water, hydrogen and carbon dioxide: burning splint for oxygen, hydrogen or carbon dioxide, and lime water for carbon dioxide. Investigate mass of reactants and products in a closed system to deduce Law of Conservation of matter Use the law of conservation of matter to explain the need for balancing equations Write and balance chemical equations predicting product(s) in a reaction using the reference tables Write and balance ionic & net ionic equations** Investigate variables that affect the rate of a reaction (nature of reactants, concentration, surface area, and temperature Explain that nature of reactants can refer to their complexity and the number of bonds that must be broken and reformed in the course of reaction Explain collision theory and relate theory to variables that affect the rate of a reaction Define and explain the role of a catalyst in a chemical reaction Interpret potential energy diagrams for endothermic and exothermic reactions including reactants, products, and activated complex Temperature change – Tie to endothermic/exothermic reaction. Express ΔH as (+) for endothermic and (–) for exothermic Unit Vocabulary 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Required Vocabulary reactant product precipitate aqueous coefficient collision theory catalyst activation energy exothermic endothermic 11. 12. 13. 14. 15. 16. 17. 18. Other Vocabulary synthesis decomposition single replacement double replacement combustion hydrocarbon carbohydrate activity series