Study sheet for chapter 8c/d Know the rules for oxidation numbers
advertisement

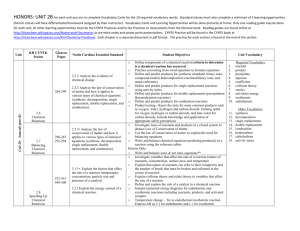
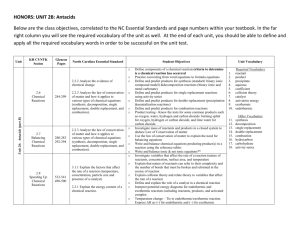
Study sheet for chapter 8c/d Know the rules for oxidation numbers and how to write balanced formulas. (pp.182 – 3 and p.196-7) Give formulas for the following compounds: a. Iron (III) oxide _____Fe2O3______ b. sulfur trioxide ____SO3______ c. ammonium bromate _____NH4BrO3________ d. potassium chloride ______KCl________ Know what activity series represent and how to use them. Predict whether the following single replacement reactions will occur in standard conditions. Base your answer on the activity series given in the text on p. 205 a. BaSO4 + Ca CaSO4 + Ba No. Ca is less active than Ba. b. BaCl2 +Br2 BaBr2 + Cl2 No. Br is less active than Cl c. Ni(OH)2 + Mg Mg(OH)2 + Ni Yes. Mg is more active than Ni d. Al2(SO4)3 + 2Fe Fe2(SO)4 +2Al No. Fe is less active than Al Know what a reactant is and what a product is and where they appear in chemical equations. (p. 196) Know the special symbology on pp. 198-99 and how to use them in equations. Know the rules for replacement in single replacement reactions (pp. 204 – 206). What is a catalyst? p.199 What is the law of conservation of mass and energy and how does it apply to chemical equations? p.196 What is a precipitate? p.198 Practice balancing equations by doing the review questions 33a. – m. on pp. 212 – 13 a. 2BaO2(s) ∆ 2BaO(s) + O2 - decomposition b. 2Li(s) + 2H2O(l) ∆ 2LiOH(s) + H2(g) – single replacement c. 2H2O2(l) + sunlight 2H2O(l) + O2(g) – decomposition d. 2Na(l) + H2(g) 2NaH(s) - synthesis e. 2H2SO4(l) ∆ 2H2O(l) + 2SO2(g) + O2(g) - decomposition f. N2(g) + 3H2(g) 2NH3(g) – synthesis g. already balanced – synthesis h. CH4(g) C)s) + 2H2 – decomposition i. SiO2(l) + 2C(s) Si(l) + 2CO(g) – single replacement ∆ j. Si(s) + 2Cl2(g) SiCl4 (l) - synthesis ∆ k. SiCl4(g) + 2H2(g) Si(s) + 4HCl(g) + Si(s) – single replacement l. Ba(s) + H2SO4(l) BaSO4(s) + H2 (g) – single replacement m. Mg(OH)2(aq) + 2HCl(aq) MgCl2(aq) +2H2O(l) – double replacement Know the four types of reactions (pp. 204 – 207) and how to recognize them from a chemical formula. Practice recognizing reaction types by telling whether each of the reactions in question 33. a-m was a synthesis, decomposition, single replacement or double replacement reaction. Know what balanced equations can and cannot indicate (p. 196 and p. 201). Remember the diatomic elements (Mr. H. BrIClFON). Recognize the common oxyanions and their charges (table 8-6 p.191)