Name: Date: Block: Types of Chemical Reactions A chemical
advertisement

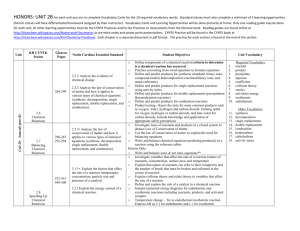
Name: Date: Block: Types of Chemical Reactions A chemical reaction is a process that is usually described by a chemical change in which the starting materials (reactants) are different from the products. Chemical reactions tend to involve movement of electrons, leading to the formation and breaking of chemical bonds. There are several different types of chemical reactions and more than one way of classifying them (chemistry.about.com, types of chemical reactions). We can classify reactions based on their reactants and what they produced and/or by the energy required or released by the reaction. There are 6 different types of reactions, but we will be discussing and using 5. Part 1: Types of Reactions 1. Synthesis reactions: What do you think “synthesis” means? What do you think a synthesis reaction does? 2. Decomposition reactions: What do you think “decomposition” means? What do you think a decomposition reaction does? 3. Single replacement reactions: What do you think occurs in a single replacement reaction? 4. Double replacement reactions: What do you think occurs in a double replacement reaction? Part 2: Examples and more information of reaction types 1. Synthesis reaction: Synthesis means to create something, so in a synthesis reaction, 2 separate reactants are combined to make 1 new thing. Does this product have the same chemical or physical properties as the starting material? o Examples: A+B 2Na + I2 2N2 + O2 AB 2NaI 2 N2O What do you notice about the reactants (the number and organization)? What do you notice about the products (the number and organization)? 2. Decomposition reaction: Decomposition means to break down (think about food webs from 7 th grade. Fungi, like mushrooms, are decomposers, what do they do?) In a decomposition reaction, the reactants start out as one material and after the reaction are broken apart. Do the reactants and products have the same physical or chemical properties? o Examples: AB 2 KClO3 2 H2O A + B 2 KCl + 3 O2 2 H 2 + O2 What do you notice about the reactants (the number and organization)? What do you notice about the products (the number and organization)? 3. Single replacement: A substitution reaction. A single element switches places with an element in another compound. o Example: A + BC AC + B 2 KI + Cl2 2KCl + I2 Zn + 2 HCl ZnCl2 + H2 4. Double replacement: 2 compounds switch bonds or ions to form different compounds. o Example: o AB + CD AD + BC o SiO2 + 4 HF SiF4 o PCl5 5HCl + H3PO4 + 4 H2O + 2H2O Part 3: Energy in Reactions We can look at how a reaction uses energy. Reactions will behave in one of two ways. 1. Endothermic—These reactions require energy. You have to continually add energy to these reactions to make them work. These reactions feel cold and if you measure the temperature, it drops as the reaction progresses. Δ H means the change in the energy. 2. Exothermic—These reactions give off energy. The energy at the beginning is higher than the energy at the end. Once the reaction is complete, it stops giving off energy. These reactions feel warm and if you measure the temperature, it rises as the reaction progresses. Practice: Balance each type of reaction 1. __2__H2 + 2. __2__Fe + ____O2 __3__Cl2 __2___H2O __2___FeCl3 3. ____S8 + __8__O2 __8___SO2 4. ____P4 + __5___O2 __2___P2O5 5. _2____NaClO3 __2___NaCl 6. __2___Al2O3 _4___Al + 7. __2___KClO3 8. ____NH4OH + __3__O2 __3___O2 __2__KCl + __3___O2 ____NH3 + _____H2O balanced 9. __2__AlBr3 + __3__Cl2 10. __2___Na + __2___H2O 11. _____Cu + __2___AgNO3 12. ______Zn + ___2___HCl 13. __3__Pb(NO3)2 + __2__AlCl3 14. ____Al2(SO4)3 + __3___Ca(OH)2 __2__AlCl3 + __3___Br2 ___2__NaOH + _____H2 _____Cu(NO3)2 + __2__Ag _____ZnCl2 + ______H2 __3__PbCl2 + __2___ Al(NO3)3 __2___ Al(OH)3 + __3___CaSO4 15. _2___AlI3 + __3___HgCl2 16. __3__AgNO3 + _____K3PO4 _2___AlCl3 + __3___HgI2 ____Ag3PO4 + __3__KNO3 17. Which of the above reactions are single replacement reactions? How do you know? 9-12 because it has one element replacing another single element in a compound 18. Which of the above reactions are decomposition reactions? How do you know? 5-8 because you start with one material & it breaks down into two or more 19. Which of the above reactions are synthesis reactions? How do you know? 1-4 because you start with two materials and they combine to form 1 material 20. Which of the above reactions are double replacement reactions? How do you know? 12-16 because 2 compounds combine by switching the first elements in both compounds Practice 2: Identify the following graphs and statements as endothermic or exothermic reactions. 1. My reaction started out with 50 J of energy, but I had to continually add energy to the reaction to make it work. endothermic 2. The temperature of my beaker increased. exothermic 3. My icepack works by combining chemicals. This is a ___endothermic___________________ reaction. 4. The reaction had 600 J of energy and once it was complete, there was 50 J of energy. 5. _exothermic__________________ 6.__endothermic____________