North Haven Public Schools Curriculum
advertisement

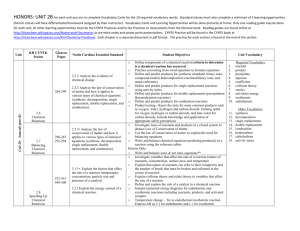
North Haven Public Schools Curriculum Unit Title/ focus: Chemical Reactions and Equations Textbook: Prentice Hall Chemistry – Chap. 11, 18.1 Designer Names (s): Larry Fabianski, Marc Horowitz, Julie Nichols Subject Area: Chemistry L2/L3 Grade Level: 10, 11, 12 Estimated Amount of Instructional Time: 3 weeks Connecticut State Standards Conservation of Matter and Stoichiometry The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. Chemical reactions can be described by writing balanced equations. Reaction Rates Chemical reaction rates depend on factors that influence the frequency of collision of reactant molecules. The rate of reaction is the decrease in concentration of reactants or the increase in concentration of products with time. Reaction rates depend on factors such as concentration, temperature and pressure. Catalysts play a role in increasing the reaction rate by changing the activation energy in a chemical reaction. Big Ideas Enduring Understandings Chemical equations must be balanced using coefficients so that they obey the Law of Conservation of Mass. There are five general types of chemical equations/reactions. The number and identity of the reactants can be used to determine reaction type and predict possible products. A net ionic equation only expresses particles involved in a reaction. The formation of a precipitate can be predicted by the use of the general rules for the solubility of ionic compounds. There are a multitude of ways to change the rate of a reaction. All chemical reactions undergo a change in energy (lose or gain). Essential Questions What are the five types of chemical reactions? How can we predict the possible products of a reaction? What are the factors that affect the rate of a reaction? How is collision theory related to the rate of chemical reactions? What is the difference between exo- and endothermic reactions? What Students Should Know and Be Able to Do (Skills and Knowledge) Prerequisite Concepts/Skills Students will… Be able to name and write formulas for compounds. Determine the number of atoms of each element present in a formula. Knowledge Students will… Recognize five types of chemical reactions. Explain the difference between an Exothermic reaction and an Endothermic reaction. Understand the Law of Conservation of Mass. Understand the factors that influence the rate of a chemical reaction. Skills Students will… Predict the products of a chemical reaction. Write and balance chemical equations. Use activity series and solubility rules to determine if a reaction will occur. Be able to construct energy diagrams. Vocabulary balanced chemical equation coefficient single replacement endothermic catalyst reactant synthesis (combination) double replacement exothermic inhibitors product decomposition combustion activation energy energy diagrams Pacing Guide Unit 7: Chemical Reactions and Equations – 8 blocks Chemical equations – 1 block o Word, Formula (chemical) equations o Balancing equations Types of Reactions – 1 blocks o Synthesis, Decomposition, Single replacement, Double replacement and Combustion Predicting Products – 1 block o Synthesis, Decomposition and Combustion o Single and Double replacement with use of activity series and solubility rules Rates of Reactions – 1 block o Factors that affect rates o Energy Diagrams (Exo-, Endothermic reactions, etc.) LABS: Chemical Reactions Laboratory (2 block) Assessment Evidence Required Assessments: Two quiz End of Unit Test Supplemental Assessments: Lab reports Lab activities: Chemical Reactions Laboratory