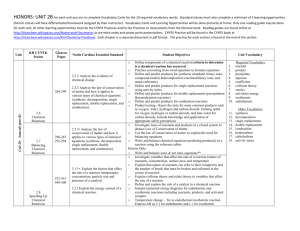
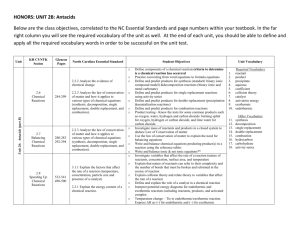
Unit 2B Antacids At the end of this unit, the student will
advertisement
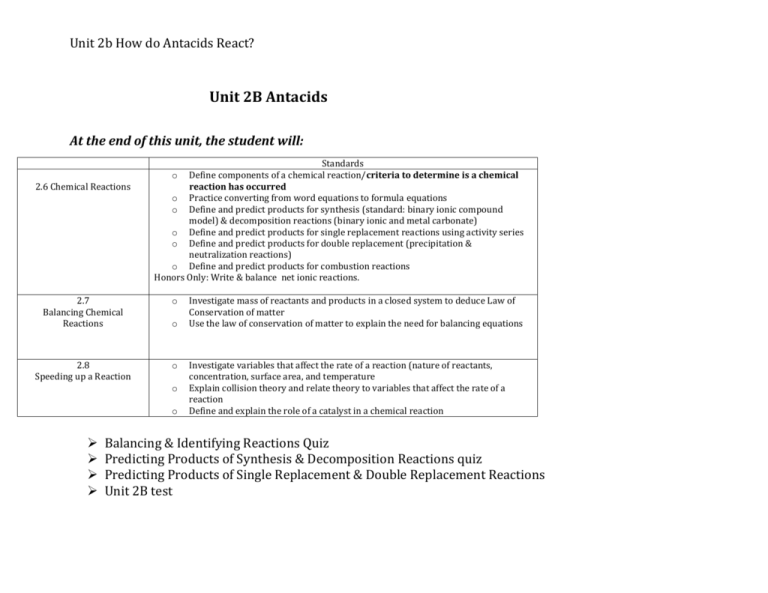
Unit 2b How do Antacids React? Unit 2B Antacids At the end of this unit, the student will: 2.6 Chemical Reactions Standards o Define components of a chemical reaction/criteria to determine is a chemical reaction has occurred o Practice converting from word equations to formula equations o Define and predict products for synthesis (standard: binary ionic compound model) & decomposition reactions (binary ionic and metal carbonate) o Define and predict products for single replacement reactions using activity series o Define and predict products for double replacement (precipitation & neutralization reactions) o Define and predict products for combustion reactions Honors Only: Write & balance net ionic reactions. 2.7 Balancing Chemical Reactions o 2.8 Speeding up a Reaction o o o o Investigate mass of reactants and products in a closed system to deduce Law of Conservation of matter Use the law of conservation of matter to explain the need for balancing equations Investigate variables that affect the rate of a reaction (nature of reactants, concentration, surface area, and temperature Explain collision theory and relate theory to variables that affect the rate of a reaction Define and explain the role of a catalyst in a chemical reaction Balancing & Identifying Reactions Quiz Predicting Products of Synthesis & Decomposition Reactions quiz Predicting Products of Single Replacement & Double Replacement Reactions Unit 2B test Unit 2b How do Antacids React?