Supplementary Material (doc 82K)
advertisement

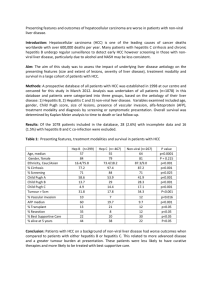
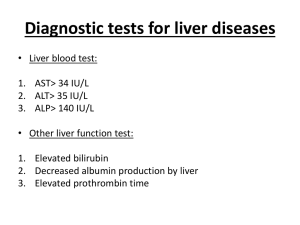
Wolf et al., Supplemental Material Etiologies of inflammation-driven carcinogenesis Besides chronic inflammation - a newly added hallmark of cancer - evading apoptosis, self-sufficiency in growth signals, insensitivity to anti-growth signals, tissue invasion and metastasis, limitless replicative potential and sustained angiogenesis are the hallmarks of cancer Chronic inflammation can be caused by viruses, such as hepatitis B and C viruses (HBV, HCV) which drive chronic hepatitis leading to hepatocellular carcinoma (HCC) (Llovet et al., 2003) or by pathogenic bacteria, such as Helicobacter pylori, which promotes gastric inflammation thereby increasing the risk of developing gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma (Cover and Blaser 2009). Infections with parasites (e.g. schistosoma) can also favor chronic inflammation consequently driving bladder carcinoma, liver cancer or colorectal cancer development (Mostafa et al., 1999). Moreover, it has been reported that autoimmune diseases accompanied by chronic inflammation contribute to cancer development: Autoimmune hepatitis is involved in HCC development (Nishiyama et al., 2004) and inflammatory bowel diseases (e.g. ulcerative colitis, Crohn’s disease) can precede colon cancer (Pohl et al., 2000). Furthermore, primary sclerotizing cirrhosis can lead to cholangiocellular carcinoma (CCC) (Maggs and Chapman 2008). Finally, in addition to pathogenic and autoimmune mediators, chronic or acute intoxication (e.g. alcohol, drug abuse, aflatoxin-b) can cause inflammation-induced carcinogenesis (Fan and Farrell 2009). Hepatic LT R signaling and pathogen infection LT has been demonstrated to directly act on hepatocytes expressing high levels of LTR but little LT (Browning and French 2002; Haybaeck et al., 2009): T-cell-derived LT and LIGHT signaling to hepatocytes was demonstrated to control lipoprotein homeostasis (Lo et al., 2007) and LTR signaling was shown to be important for liver regeneration through T-cell-derived LT expression (Tumanov et al., 2009). Ruddell and colleagues demonstrated that LTR signaling regulates hepatic stellate cell function and wound healing (Ruddell et al., 2009). Thus, hepatic LTR signaling seems to control liver homeostasis, in both health and disease. Indeed pharmacological inhibition of LTR signaling in the context of pathogen infections has recently demonstrated that virus-, bacteria- and concavalin A-induced liver injury could be significantly reduced (An et al., 2006; Anand et al., 2006; Puglielli et al., 1999). An involvement of LTR signaling in the host response to liver infection was studied in the case of HCV in vivo and in vitro. Increased LT expression in human livers was found to correlate with HCV-infection especially in liver progenitor (oval) cells but also in small portal hepatocytes as well as immune cells (Lowes et al., 2003). In addition, LT was reported to be upregulated as a consequence of HBV X protein expression in liver cell lines (Lee et al., 2005) and siRNA knock-down of various components of the LTR signaling pathway (e.g. LT; Rel A) was shown to interfere with HCV replication in vitro (Ng et al., 2007). Indeed, several reports point towards an interaction of the HCV core protein with the LTR, leading to the modulation of the LTR-signaling pathway (Chen et al., 1997; Matsumoto et al., 1997; Zhu et al., 1998). The causal link between HCV- or HBV-infection, sustained LTR signaling and liver cancer development will be further discussed in the main section of this review. References An MM, Fan KX, Cao YB, Shen H, Zhang JD, Lu L et al., (2006). Lymphtoxin beta receptor-Ig protects from T-cell-mediated liver injury in mice through blocking LIGHT/HVEM signaling. Biol Pharm Bull 29: 2025-2030. Anand S, Wang P, Yoshimura K, Choi IH, Hilliard A, Chen YH et al., (2006). Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest 116: 1045-1051. Browning JL, French LE (2002). Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol 168: 5079-5087. Chen CM, You LR, Hwang LH, Lee YH (1997). Direct interaction of hepatitis C virus core protein with the cellular lymphotoxin-beta receptor modulates the signal pathway of the lymphotoxin-beta receptor. J Virol 71: 9417-9426. Cover TL, Blaser MJ (2009). Helicobacter pylori in health and disease. Gastroenterology 136: 18631873. Fan JG, Farrell GC (2009). Prevention of hepatocellular carcinoma in nonviral-related liver diseases. J Gastroenterol Hepatol 24: 712-719. Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO et al., (2009). A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 16: 295-308. Lee SH, Park SG, Lim SO, Jung G (2005). The hepatitis B virus X protein up-regulates lymphotoxin alpha expression in hepatocytes. Biochim Biophys Acta 1741: 75-84. Llovet JM, Burroughs A, Bruix J (2003). Hepatocellular carcinoma. Lancet 362: 1907-1917. Lo JC, Wang Y, Tumanov AV, Bamji M, Yao Z, Reardon CA et al., (2007). Lymphotoxin beta receptordependent control of lipid homeostasis. Science 316: 285-288. Lowes KN, Croager EJ, Abraham LJ, Olynyk JK, Yeoh GC (2003). Upregulation of lymphotoxin beta expression in liver progenitor (oval) cells in chronic hepatitis C. Gut 52: 1327-1332. Maggs JR, Chapman RW (2008). An update on primary sclerosing cholangitis. Curr Opin Gastroenterol 24: 377-383. Matsumoto M, Hsieh TY, Zhu N, VanArsdale T, Hwang SB, Jeng KS et al., (1997). Hepatitis C virus core protein interacts with the cytoplasmic tail of lymphotoxin-beta receptor. J Virol 71: 1301-1309. Mostafa MH, Sheweita SA, O'Connor PJ (1999). Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev 12: 97-111. Ng TI, Mo H, Pilot-Matias T, He Y, Koev G, Krishnan P et al., (2007). Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology 45: 14131421. Nishiyama R, Kanai T, Abe J, Hara R, Watahiki Y, Sakaguchi T et al., (2004). Hepatocellular carcinoma associated with autoimmune hepatitis. J Hepatobiliary Pancreat Surg 11: 215-219. Pohl C, Hombach A, Kruis W (2000). Chronic inflammatory bowel disease and cancer. Hepatogastroenterology 47: 57-70. Puglielli MT, Browning JL, Brewer AW, Schreiber RD, Shieh WJ, Altman JD et al., (1999). Reversal of virus-induced systemic shock and respiratory failure by blockade of the lymphotoxin pathway. Nat Med 5: 1370-1374. Ruddell RG, Knight B, Tirnitz-Parker JE, Akhurst B, Summerville L, Subramaniam VN et al., (2009). Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology 49: 227-239. Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B et al., (2009). T cellderived lymphotoxin regulates liver regeneration. Gastroenterology 136: 694-704 e694. Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C et al., (1998). Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol 72: 3691-3697.