Solubility and Concentration Review
advertisement
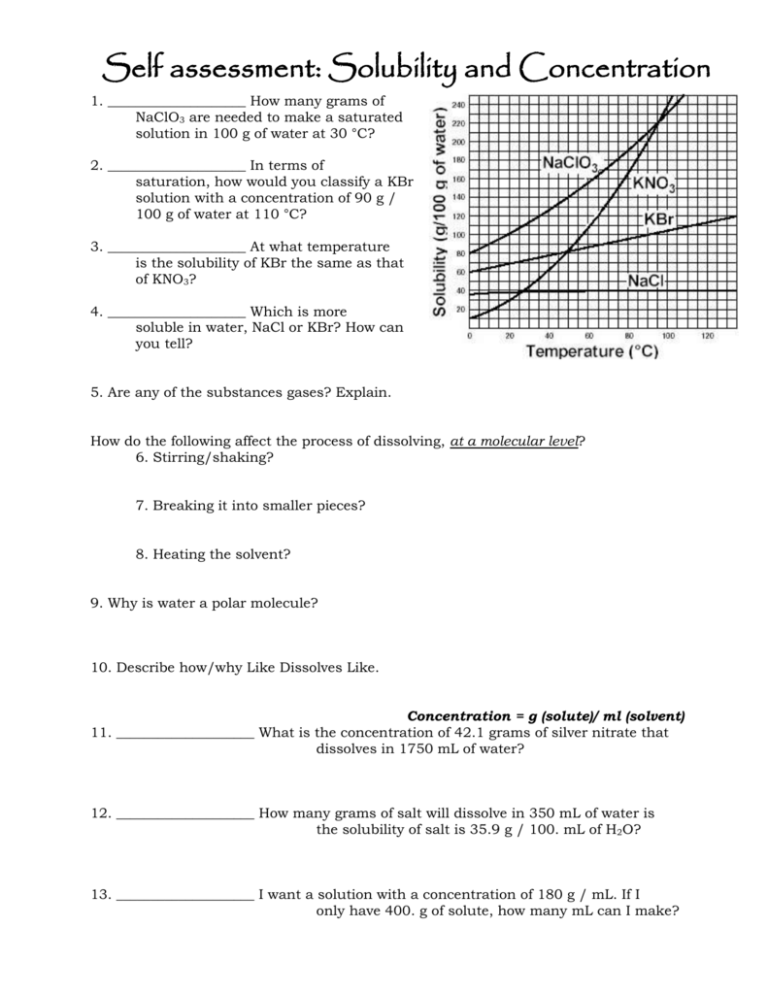
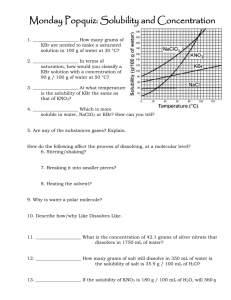
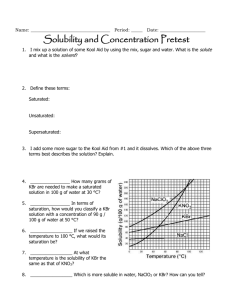
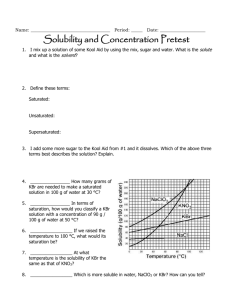
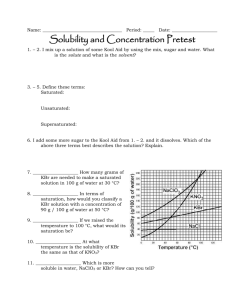
Self assessment: Solubility and Concentration 1. ____________________ How many grams of NaClO3 are needed to make a saturated solution in 100 g of water at 30 °C? 2. ____________________ In terms of saturation, how would you classify a KBr solution with a concentration of 90 g / 100 g of water at 110 °C? 3. ____________________ At what temperature is the solubility of KBr the same as that of KNO3? 4. ____________________ Which is more soluble in water, NaCl or KBr? How can you tell? 5. Are any of the substances gases? Explain. How do the following affect the process of dissolving, at a molecular level? 6. Stirring/shaking? 7. Breaking it into smaller pieces? 8. Heating the solvent? 9. Why is water a polar molecule? 10. Describe how/why Like Dissolves Like. Concentration = g (solute)/ ml (solvent) 11. ____________________ What is the concentration of 42.1 grams of silver nitrate that dissolves in 1750 mL of water? 12. ____________________ How many grams of salt will dissolve in 350 mL of water is the solubility of salt is 35.9 g / 100. mL of H2O? 13. ____________________ I want a solution with a concentration of 180 g / mL. If I only have 400. g of solute, how many mL can I make? Self assessment: Solubility and Concentration 112g 1. _____ _______ How many grams of NaClO3 are needed to make a saturated solution in 100 g of water at 30 °C? unsaturated_ In terms of 2. __ saturation, how would you classify a KBr solution with a concentration of 90 g / 100 g of water at 110 °C? (It’s holding less than the maximum it can….) 50 C o 3. ____ ________ At what temperature is the solubility of KBr the same as that of KNO3? KBr_______ Which is more soluble in water, NaClO3 or KBr? How can you 4. _____ tell? You can dissolve more at all temperatures, so it is more soluble (tell me more than “the line is higher”…tell me what that means when the line is higher!) 5. Are any of the substances gases? Explain. No. If they were, the line would go down as temp. goes up. How do the following affect the process of dissolving, at a molecular level? 6. Stirring/shaking? Moves molecules away, so more room for interactions with solute. 7. Breaking it into smaller pieces? More surface area for interactions between water and solute molecules. 8. Heating the solvent? Molecules of solute and solvent move faster and interact more 9. Why is water a polar molecule? Uneven distribution of electrons…one side has a little positive charge (+) and one side is a little negative (-) 10. Describe how/why Like Dissolves Like. Polar things will dissolve in polar solvents, nonpolar solutes will dissolve in nonpolar solvents. Because the charges in a polar molecule will pull apart the other if it also has charges. (polar will pull apart polar, but not nonpolar) Concentration = g (solute)/ ml (solvent) .024 g/mL___ What is the concentration of 42.1 grams of silver 11. __ nitrate that dissolves in 1750 mL of water? 42.1 g 1750 mL x = .0240571 130 g___ How many grams of salt will dissolve in 350 mL of water is 12. ___ the solubility of salt is 35.9 g / 100. mL of H2O? 35.9 g 100mL = 35.9 x 350 = 100 x x = 125.65 xg 350mL 12565 = 100 x 2.2 mL__ I want a solution with a concentration of 180 g / mL. If I 13. __ only have 400. g of solute, how many mL can I make? 180 = 400/x 180x = 400 x = 400/180 x = 2.22222222