Solutions - Chemistry
advertisement

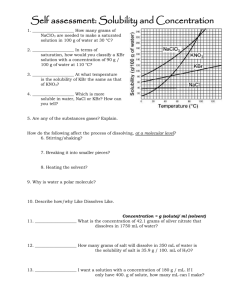
Solutions Water Water is a bent molecule and is polar, and the molecules are held together through hydrogen bonding. Properties of Water 1. 2. 3. High Surface Tension – acts like a “thin” skin on the surface. Low vapor pressure & High Boiling Point – needs a lot of energy/heat to make it evaporate or boil High Specific Heat – takes a lot of heat to change its temperature WATER! It’s good for! You! I think! Washcloth Can you cry in space? Suspensions (large particles) filtered out with filter paper, and They can be ____________ they will _________ _____ settle out by gravity. scatter These particles will also ______________ light shined through the mixture so the beam can be seen. vinegar and oil salad dressing Colloids (small particles) filtered They cannot be ___________ nor do they__________ _____ settle out , but they are still big enough scatter to ___________ a beam of light. What is a solution? A solution is a homogeneous mixture (same throughout) A solution can be a gas, liquid or solid. Examples: Air = O2 in N2 Kool-aid = Sugar in water Brass = mixture of copper in zinc Solutions Two Parts to a Solution: Solvent the dissolving medium which is 1) ____________typically a liquid or the substance in greater amount when 2 similar phases are mixed 2) _____________ - the substance that dissolves Solute Hello again… Aqueous Solutions Solutions with water as the solvent. Kool-aid = SUGAR in water Does sugar “disappear” or dissolve in water? Does salt “disappear” or dissolve in water? The dissolving questions have to do with polarity… Is water polar? Stuff that dissolves in Water 1. Polar Molecules – sugar is a polar molecule because of the many polar –OH groups. 1. Ionic Substances – salt is an ionic substance. Ionic substances: cation + anion Stuff that doesn’t dissolve in Water 3. Nonpolar Molecules – petroleum is nonpolar because of all the nonpolar C-H bonds in the molecule. The petroleum instead floats on water. “Like dissolves like” This phrase means that polar solvents dissolve __________ polar solutes and nonpolar solvents dissolve ___________ nonpolar solutes. Polar dissolves Polar Nonpolar dissolves Nonpolar You try “Like dissolves Like” Would rubbing alcohol (polar) dissolve in nonpolar oil? Would nonpolar iodine (I2) dissolve in oil? Solutions & Electricity __________________Electrolyte a solution that conducts electricity. Nonelectrolyte __________________a solution that does not conduct electricity. What Determines if a Solution will Conduct Electricity? charged ______________ particles A solution will conduct if there are ____________ ions (or _________), in the solution. Most typical electrolytes: IONIC SOLUTIONS Example: Salt (NaCl) Some solution vocab… Miscible • _______________ : two liquids that can dissolve in each other alcohol in water Example: ____________ • _________________ : the liquids don’t mix Immiscible Oil and water Example: _____ Exit Slip 1. If something dissolves in water, would it be a polar or nonpolar substance? 2. What would make a better electrolyte: Li3N or SF6? 3. My name is monkey…. What is my favorite smoothie? Answers to Solutions Practice f, e, a, b, c, d 2. Solvent = Water; Solute = Coffee Beans 3. Heterogeneous – looks different throughout; Homogeneous – looks the same throughout (pg 39) 4. Polar dissolves polar; nonpolar dissolves nonpolar. Chemically like substances dissolve each other 5. Grease stain must be nonpolar because it does not dissolve in water and water is a polar substance; water just spreads the oil (in real life soap would be better and safer soap has the ability to dissolve both polar and nonpolar) 6. Miscible choc syrup is mixable with milk 7. E, N, N, E, E 8. Gasoline – maybe unconventional, but can dissolve the oil (it’s why it’s hard to wash oil off with water) 9. Unsaturated (it’s in the last section of your notes) 1. Solubility solute can dissolve Solubility tells us how much _________ in a certain amount of _____________ at a particular solvent temperature and pressure to make a saturated “__________________” solution. _________________ Saturated solutions: a solution that contains as much solute as will dissolve at that temperature ________________ Unsaturated solutions: a solution in which more solute can dissolve than is already dissolved at that temp Factors that Affect Solubility: Temperature (abnormal behavior) Increase temperature, increase solubility. On the solubility curve, the lines indicate the concentration saturated solution. of a ____________ Values on the graph _____________ below a curve represent unsaturated solutions. Example on Reading the Graph: -Find the curve for KClO3 At 30°C approximately 10 g of KClO3 will dissolve in 100g of water. If the temperature is increased to 80°C, approximately ______ 40 g of the substance will dissolve in 100g (or 100mL) of water. 1. What mass of KNO3 will dissolve in 100 mL of water at 70°C? ________. 2. What is the solubility of KCl at 10°C? ______ g per 100 g H2 O 3. a solution that contains 70g of NaNO3 at 30°C (in 100 mL H2O) Factors that Affect Solubility: ------------------------------------------------- Question: What are 3 things you can do to get a solid to dissolve more rapidly in a liquid? 1) Heat up the solution. 2) Stir the solution. 3) Crush the solid. Solubility Curves Answers 1) 2) 3) 4) 5) a. ~44 g b. 40 g c. 70 g d. NaCl (all answers g per 100 g H2O) a. 34 g b. ~14 g c. 5 g Unsaturated 1. sat, - 2. sat, 3. unsat, 60 g 4. unsat, 35g KClO3 Since we’re working with graphs – an approximation for numbers. Be in the ballpark!! Exit Slip 1. 2. How many more grams would it take to saturate a solution a solution that contains 65 grams of NaNO3 at 50 degrees Celsius? (how much more solute can dissolve?) If you had to name a penguin for a short story, what would you name the penguin? Answers to Solutions Practice 2 1) Hydrogen Bonding 3) B 4) C 5) A, B 2) A lot 6) C 7) C 8) B 9) A 10) A 10.5) A, B, C 11) Unsaturated 12) A. Unsaturated b. Around 34 degrees C (the saturation point for NaNO3 of 100 grams is at 35 degrees so above the saturation line is when the solid cannot dissolve anymore in the solute, 100 grams is above the solute line around 34). 14) a. No b. Unsat c. Copper Sulfate Exit Slip 1. 2. 3. Why can a mosquito can stand on water (think about properties of water) What type of mixture (suspension/colloid/solution) would clouds be considered? If you found out you could fly, what would be the first thing you would do? Exit Slip 1. Explain why a mosquito can stand on water (think about properties of water) 2. If you found out you could fly, what would be the first thing you would do? Molarity & Concentrations It’s a hot day.You took a sip of your lemonade. And it tasted wayyyyyy too sweet. What can you do to make your lemonade taste better? Measuring the Concentration of a Solution Concentration how much solute is dissolved in a certain ___________________: amount of solvent at some given temperature and pressure. Qualitative Vocabulary Dilute _______________ : contains a small amount of solute Concentrated : contains a large amount of solute _______________ concentrated dilute Molarity Expression of concentration of a solution. moles of solute Molarity (M) = liters of solution mol L 1 mol of solute per liter of solution is written as 1.0 (that is one molar) moles Molarity X Liters M Making a Solution of a Required Concentration # of moles ÷ # of liters = Molarity Example What is the molarity of 2.0 moles of NaCl in 4L of water? 1000 mL = 1 L Example What is the molar concentration of an aqueous NaCl solution when 25.0 grams are dissolved in water to make 500 mL of solution? Making Dilutions Dilution: Making a solution _______ less concentrated by ________ adding more ___________ . solvent Why dilutions??!! “To save time and space in a lab, solutions are purchased in concentrated form (stock solutions). So, water (or another solvent) is added to achieve the molarity of a particular solution” Important: When diluting acids: “Do like you otta, add acid to watta” (add acid to base) Equation for Dilutions M1V1=M2V2 M1 -- the initial concentration of the solution. V1 -- the initial volume of the original solution that is going to be diluted with water. M2 -- the final concentration of the solution after it’s diluted with water. V2 -- the total volume of the final solution after it has been diluted with water. Making Dilutions M1xV1=M2xV2 Practice Problem: 1) The science department buys HCl in large bottles that have a concentration of 12 Molar. The science teacher then dilutes the acid for labs. How would the teacher make 2.0 liters of a 2.5 M HCl solution from this “stock” solution? ( 12 M ) ( V1 ) = ( 2.5 M ) ( 2.0 L ) V1 = 0.417 L Take 0.417 L of the 12 M solution and add enough water to make a final volume of 2.0 liters. Making Dilutions M1xV1=M2xV2 2) What is the final concentration of a sugar solution if water is added to dilute 500 mL of a 2.5 M sugar solution to make a 800 mL solution? ( 2.5 M ) ( 500 mL) = ( M2 ) ( 800 mL) M2 = 1.56 M