Nuclear Chemistry Teacher Packet
advertisement

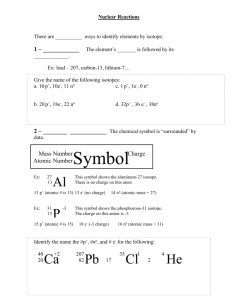
Nuclear Chemistry I: Definitions 1) Nuclear Chemistry: deals with the changes in the nucleus of the atom 2) Transmutation: the conversion of one element to another by means of a nuclear change. 3) Natural Radioactivity: the spontaneous breakdown of atomic nuclei, accompanied by the release of some form of radiation. 4) Induced radioactivity: artificial radioactivity produced by bombarding the nuclei of stable atoms with high energy particles, thereby producing radioactive atoms. 5) Radioisotopes: an isotope of an element that emits radiation. *All elements that have an atomic number than 83 have no stable isotopes! 6) Alpha particles: the nucleus of helium 7) Beta particles: an electron 8) Gamma rays: high frequency electromagnetic waves similar to x-rays, but of greater frequency 9) Positron: a positive subatomic particle with the same mass at the electron. Its charge is equal in quantity but opposite in sign to that of the electron. 10) Nuclear reaction: reaction involving a change in the nuclear contents of one or more atoms. In nuclear reactions, mass is converted to energy. 11) Nuclear fission: the splitting of an atomic nucleus into two smaller nuclei that are approximately equal in mass. 12) Fission reactors: a nuclear reactor used to control fission reactions. 13) Fusion reaction: a nuclear reaction in which two or more light nuclei combine to form a single nucleus. 14) Moderators: the substance used in a nuclear reactor to slow down the velocity of the neutrons. 15) Half-life: the time it takes for one half of the atoms in a given sample to decay. 16) Radioactive dating: the use of half-lives of radioisotopes in determining the age of the earth, ancient relics, and similar objects. 17) Tracer: Any radioisotope used to follow the path of a material in a system. II: Natural Radioactivity: the spontaneous breakdown of atomic nuclei, accompanied by the release of some form of radiation A) Transmutation: the conversion of one element to another by means of a nuclear change C) Types of Radioactive emissions (emanation) -All differ in mass, size and charge 4 2 1) Alpha Particle: ___positive 2 charge_________ ___helium nucleus____________ ____mass of 4amu Example: 226 Ra 88 222 Rn 86 2) Beta Particle: + 4 He 2 0 -1 __negative charge____________ __acts like a high speed electron __no mass______________________ Example: 235 U 92 235 Np 93 + 0 -1 3) Gamma Radiation (NOT a Particle) __no charge____________________ __acts like a high speed x-ray but with a lot more energy __no mass______________________ 4) Positron Particle: 0 +1 __positive 1 charge_________ __acts like positive electrons __no mass__________________________ Summary of Radioactive emanations: Particle Symbol Mass Charge Relative Penetrating Power alpha 4 2 4amu +2 low beta 0 -1 0 -1 moderate gamma 0 0 high positron 0 +1 0 +1 moderate Separation of Radioactive emissions by charge: Positively Charged Plate Negatively Charged Plate + - + - + - + - D) Half-life: the time it takes for half of the atoms in a given sample of an element to decay *Some selected half-lives are found on Table N *All reactions on Table N are Natural Transmutation *Formulas for half-life reactions are found on Table T 1) Fraction Remaining = (1/2)n where n refers to the number of half-lives. 2) (n) = Number of Half-lives = total time half-life of isotope 3) Original Mass = Final Mass x 2n where n refers to the number of half-lives Example: What fraction remains of an original sample of I-131 at the end of 32.28 days? Half-life of I-131 is 8.07 days. Therefore 4 half-lives have occurred. (1/2)n = (1/2)4 = 1/16 Example: What is the half-life of an element that decays from 100g to 25g in 30 days? 100g50g25g = 2 half-lives Therefore one half-life equals 15 days Example: If we start with 36 g of 42K, how many grams will remain after 37.2 hours? 37.2 hours 12.4 hours = 3 half-lives 36g18g9g4.5g Half-life problems 1. If you start with 75 g of P-32, how many grams will be left after 42.9 days? 42.9 days 14.3 days = 3 half-lives 75g37.5g18.75g9.375g 2. After 4800 years, there is 2.0 g of Ra-226 remaining. What was the original mass of the sample? Original Mass = Final Mass x 2n 4800 years 1600 years = 3 half-lives Original Mass = 2.0g x 23 = 16g 3. A 40 gram sample of P-33 decays to 10 grams in 50 days. What is the half-life of P33? 40g20g10g = 2 half-lives Therefore one half life equals 25 days 4. What fraction of C-14 remains of a 1 gram sample after 17,190 years? 17190 years 5730 years = 3 half-lives Fraction Remaining = (1/2)n = (1/2)3 = 1/8 III: Artificial Radioactivity A) Artificial Transmutation: artificial radioactivity produced by bombarding the nuclei of stable atoms with high energy particles, thereby producing radioactive atoms B) Fission Reactions: the splitting of an atomic nucleus into two smaller nuclei by bombardment of a neutron (Nuclear Bomb). *Nuclear Reactors are fission reactors. Example: 235 U 92 + 10n 142 Ba 56 + 19136Kr + 3(10n) + Energy Fusion Reactions: a nuclear reaction in which _____________________________________________________________________. Requires huge amounts of pressure and extremely high temperatures. Produces much more energy than fission. Occurs naturally on the sun. (H-bomb) Example: 2 H 1 + 21H 4 He 2 + Energy IV: Uses of Radioactive Isotopes: A) Lab: to trace chemical reactions B) Industry: Radiating food to preserve by killing bacteria, mold, insect eggs C) Medicine: must have relatively short half-lives and be quickly eliminated from the body. 1) I-131 diagnosing and treating Thyroid conditions (half-life 8.07 days) 2) Co-60 emits large amounts of gamma radiation as it decays, these rays can be aimed at cancerous tumors (half-life 5.26 years) 3) Ra-226 used in treatment of certain cancers (half-life 1600 years) 4) Tc-99 used in diagnosis of brain tumors (half-life 2.13x105 years D) Geology: 1)Fossils C-14 to C-12 ratio (while alive they are in equal amounts, once an organism dies C-14 is no longer taken in) 2) Rocks U-238 to Pb-206 ratio (U-238 decays through a series of steps until it forms stable Pb-206. As time passes, the amount of U-238 decreases while the amount of Pb-206 increases). V: Balancing Nuclear Equations Steps for Balancing: 1) Due to the Law of the Conservation of Matter, whatever is on the left side of the equation must also be on the right side of the equation. 2) Add the mass numbers on the left side of the equation, this MUST equal the total mass numbers of the right side of the equation. 3) Add the atomic numbers on the left side of the equation, this MUST equal the total atomic numbers on the right side of the equation. Example: 32 S 16 + 10n 1 H 1 + 32 P 15