Investigation 34
advertisement

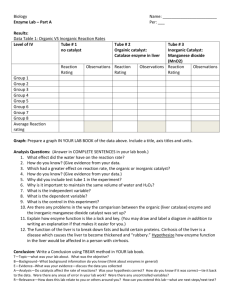
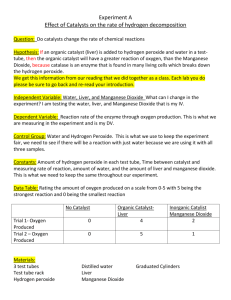
Investigation 34 Introduction Purpose Materials and Equipment Procedure Enzymes One of the distinguishing features of living organisms is the presence of organic catalysts called enzymes. Those enzymes isolated to date have been found to be proteins; most are soluble in water or a dilute salt solution. However, enzymes found in mitochondria are bound together by lipoprotein (a phospholipid-protein complex) and this makes them insoluble in water. Some enzymes consist solely of protein. Others consist of two parts, one of which is a protein, an apoenzyme, the other being made of a smaller organic molecule, a coenzyme. In some cases the second portion is a metallic ion and is known as a cofactor. Separately, the apoenzyme and the coenzyme or cofactor remain inactive, but combined they form an active functional unit known as a holoenzyme. To compare the action of catalase to a non-protein catalyst under different conditions. Materials 3% hydrogen peroxide manganese dioxide fresh or frozen liver potato ice Equipment fine clean sand stirring rod Bunsen burner or hotplate 250 mL beaker test tubes scalpel Catalytic reactions Add 2 mL of hydrogen peroxide to two test tubes. Place 0.1 g of sand in one test tube and add 0.1 g of manganese dioxide to the second tube. Observe and record the rates of reaction. What gas evolves? B. The effect of an enzyme Add 2 mL of hydrogen peroxide to each of two clean test tubes. In one place a small piece of liver and in the other a small piece of potato. Record the rates of reaction and compare these results to those with manganese dioxide. Do not discard these materials. C. Re-using an enzyme Divide the liquid portion of the previous tube in B containing the liver into two test tubes. Cut the liver from procedure B into two equal portions and add these to the two test tubes. To the first add a fresh piece of liver and to the second add 1 mL of hydrogen peroxide. Record your observations and explain the reaction in the test tube containing the fresh liver. What would happen if additional hydrogen peroxide were added to the second tube? D. Effect of particle size Place a small piece of liver in one test tube and a small piece of potato in a second. Add a pinch of sand to each tube and crush these materials with separate stirring rods. Add 2 mL of hydrogen peroxide to each tube, then observe and record the rates of these reactions. Compare the results with those of the uncrushed liver and potato in part B. tt.1 2 mL H 20 2 liver Record Results tt.2 2 mL H202 potato Record Results B. Effect of an enzyme ----------C. Re-using an enzyme E. Effect of temperature Place a small piece of liver in a test tube and heat it for 5 minutes in a boiling water bath. Add 2 mL of hydrogen peroxide to the boiled liver and record the results. Place a small piece of liver into each of two test tubes. Place one test tube in a 37'C water bath for 5 minutes and the second test tube in an ice-water bath for the same length of time. Remove both test tubes from the water baths and add 2 mL of hydrogen peroxide to each tube. Record the rates of the reactions. Observations Record your results in a chart similar to the one shown below. Rates of reaction can be designated as follows: 0 = no reaction, + = slow, ++ = moderate, +++ = fast. Reaction Rates Observations A B Rate of Reaction Interpretations Sand MnO 2 Liver - Potato Used liver + fresh liver C Used liver + H2O2 Crushed liver D Crushed potato Boiled liver E Liver at 37°C Liver at 0°C Questions 1. How do you account for the differences in the rates? 2. Can H 20 2 be broken down by catalysts other than those found in living systems? Explain your answer. 3. Describe the effect of temperature and particle size on the rate of enzyme action. 4. The body temperature of a dog is approximately 40°C. Would your results be different if you had used pieces of dog liver for this investigation? 5. Explain the difference between the rates of reaction with potato as compar ed to liver.