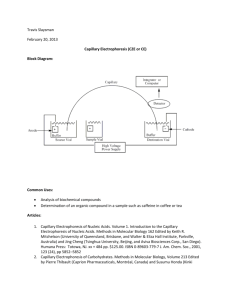
DEVELOPMENT OF CAPILLARY ELECTROPHORESIS
advertisement

DEVELOPMENT OF CAPILLARY ELECTROPHORESIS-FLUORESCENCE METHOD FOR DETERMINATION OF AMINO ACIDS IN FUNCTIONAL DRINKING SAMPLES Natta Wiriyakun*, Ajala Praneedsuranon, Duangjai Nacapricha, Rattikan Chantiwas# Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, Rama VI Rd, Rachathewi, Bangkok 10400, Thailand *e-mail: n.wiriyalun@gmail.com, #e-mail: rattikan.cha@mahidol.ac.th Abstract Development of a simple capillary electrophoresis has been investigated to determine amino acids (Lysine; Lys, Tyrosine; Tyr, Phenylalanine; Phe, Isoleucine; Ile and Glycine; Gly) in health drink samples. An in-house capillary electrophoresis system with fluorescence detection was set up and the capillary electrophoresis separation method was investigated. The optimum capillary electrophoresis separation conditions such as buffer system; buffer concentration, addition of hydroxyl propyl(methyl) cellulose, injection volume (siphoning injection time) are presented. Electrophoretic separation conditions were borate buffer (40.0 mM, pH 9.00) containing SDS (20.0 mM) and HPMC (0.1%, w/v) and applied electrical field strength of 270 V/cm. The linear calibration curves of Lys-FITC, Phe-FITC and Ile-FITC were 30-250 μM, while Tyr-FITC was linear in range of 20-250 μM, and Gly-FITC was obtained in range of 50-250 μM. Percentage of relative standard deviation (%RSD) of migration times and peak area were less than 3.2% and 4.9%, (n=3), respectively. Limit of detection (LOD) and limit of quantification (LOQ) for all amino acids were less than 29 µM and 95 µM, respectively. Determination of amino acids in health drink samples was carried out by developed method. The labeled amount of amino acids contained in the samples and the measured amounts by CE-FL method were compared. Percentage of difference was acceptable, it was less than 15%. The reasonable accuracy based on recovery study for Lys, Ile and Phe was in ranges of 80-115% (n=3). Keywords: amino acids, capillary electrophoresis, fluorescence, functional drinking samples, spectrofluorometer References 1. Poinsot VC, M.; Bouajila, J.; Gavard, P.; Feurer, B.; Couderc, F. Recent advances in amino acid analysis by capillary electrophoresis. Electrophoresis. 2012;33:14-35. 2. Herrero MGa-Ca, V.; Simo, C.; Cifuentes, A. Recent advances in the application of capillary electromigration methods for food analysis and Foodomics. Electrophoresis. 2010;31:205-18. 3. Jin LJR, I.; Li, S. Enantiomeric separation of amino acids derivatized with fluoresceine isothiocyanate isomer I by micellar electrokinetic chromatography using b- and g-cyclodextrins as chiral selectors. Electrophoresis. 1999;20:1538-45. 4. Lagane BT, M.; Couderc, F. Capillary electrophoresis: theory, teaching approach and separation of oligosaccharides using indirest UV detection. Biochemistry and Molecular Biology Education. 2000;28:251-5. 5. MacTaylor CEE, A.G. Critical review of recent developments in fluorescence detection for capillary electrophoresis. Electrophoresis. 1997;18:2279-90. 6. Tezcan FU, S.; Uyar, G.; Öztekin, N.; Erim, F. Determination of amino acids in pomegranate juices and fingerprint for adulteration with apple juices. Food Chemistry. 2013;141:1187-91. 7. Chapman SDGaPJ. Measuring Electroosmotic Flow in Microchips and Capillaries. In: Henry CS, editor. Microchip Capillary Electrophoresis: Methods and Protocols. Totowa, NJ: Humana Press Inc.; 2006. p. 187-201. 8. Weinberge R. Practical Capillary Electrophoresis. New York: Academic Press; 1995.