1.7AMINES
advertisement

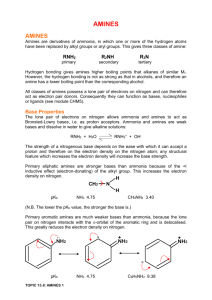
AMINES Amines are considered to be derivatives of ammonia, in which one or more of the hydrogen atoms in the ammonia molecule have been replaced by an alkyl group. Replacement of one H atom of ammonia with an alkyl group produces a PRIMARY AMINE replacement of two H atoms produces a SECONDARY AMINE replacement of all three H atoms results in a TERTIARY AMINE. H—N—H R—N—H | | | | H H H R’’’ Ammonia Primary Amine R-N—R’ R—N—R” Secondary Amine Tertiary Amine Amines are found in many biological molecules, including AMINO ACIDS, and VITAMINS, and are used in the manufacture of pharmaceuticals such as Sulfa drugs and anesthetics. The synthetic fibre, NYLON, is an amine derivative. THE NAMING OF AMINES: The IUPAC names of amines are formed by adding the prefix amino- to the corresponding alkane name of the amine. As usual, numbers are used to indicate the carbon to which the amine substituent is attached. The functional group takes priority over the alkyl group. CH3-NH2 CH3 – CH – CH2 – CH – CH2 – CH3 aminomethane | | CH3 NH2 3-amino-5-methylhexane aminobenzene For secondary or tertiary amines, the naming uses the “N” to indicate alkyl groups attached directly to the nitrogen. CH3 – CH – CH2 – CH – CH3 | CH3 | -N-CH2-CH3 \ CH2-CH3 NH – CH3 N-methyl-2-amino-4-methylpentane (2O) N,N-diethylaminobenzene QUESTIONS 1. Name and classify the following amines. a) NH2 – CH2 – CH2 – CH – CH3 b) | NH2 NH2 NH2 c) NH – CH2 – CH2 – CH2 – CH – CH – CH3 | | d) CH3–CH2-CH2-NH–CH2–CH3 CH3 CH3 2. Draw the structural formulas of four amines that have the molecular formula C3H9N (two primary, one secondary, and one tertiary amine). Classify the amines and name them. PROPERTIES OF AMINES 1. Amines have an unpleasant odours. (the odour of decaying fish is attributed to simple amines). 2. Because of the hydrogen bonding amines have higher BP than hydrocarbons of similar molar mass. Since a nitrogen—hydrogen bond is less polar than an oxygen—hydrogen bond, the hydrogen bonding in primary amines is weaker than in alcohols of related formulas. As a result, the boiling points of amines are lower than those of the related alcohols. 3. Amines are weak bases. Amines have a relatively high solubility in water because of the hydrogen bonding that occurs in primary and secondary amines. Tertiary amines do not hydrogen bond. REACTIONS Ammonia reacts with alkyl halides to produce an amine. CH3-Cl + NH3 Chloromethane ammonia CH3NH2 + aminomethane HCl hydrogen chloride If the aminomethane is reacted with more chloromethane a secondary amine will be created. Questions 3. Find the products in a reaction between chloroethane and 1-aminobutane. 4. Arrange the following molecules from lowest BP to the highest: ethanol, ethanal, aminoethane, propane.