Nomenclature!!! - What`s in a name? REMEMBER!!! When naming
advertisement
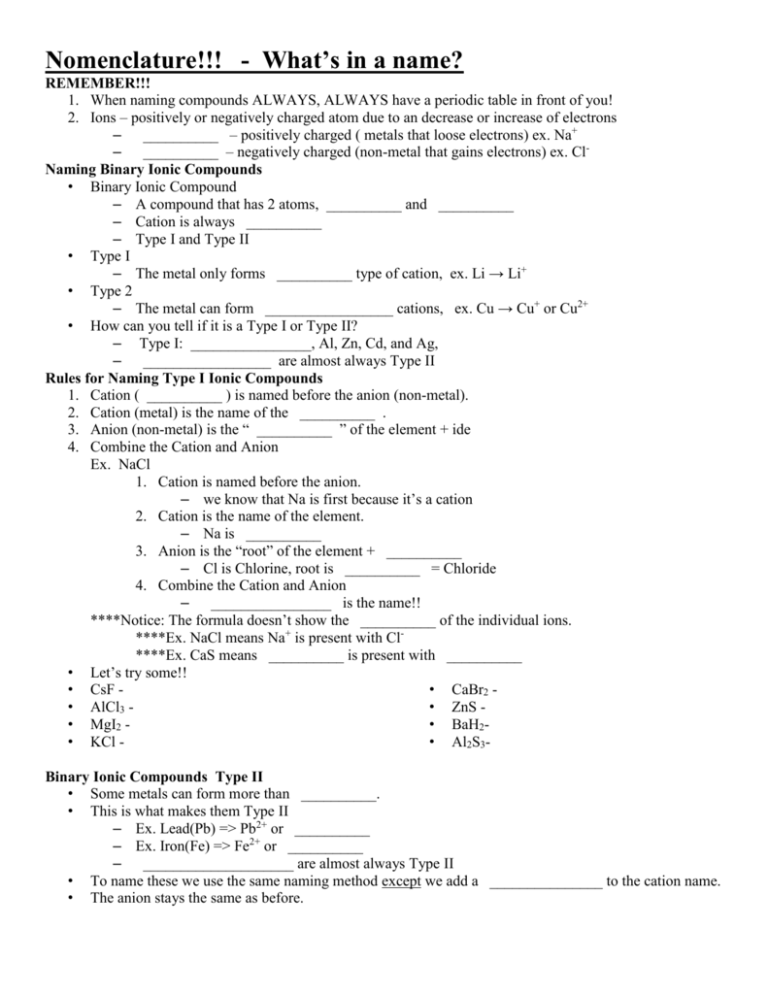
Nomenclature!!! - What’s in a name? REMEMBER!!! 1. When naming compounds ALWAYS, ALWAYS have a periodic table in front of you! 2. Ions – positively or negatively charged atom due to an decrease or increase of electrons – __________ – positively charged ( metals that loose electrons) ex. Na+ – __________ – negatively charged (non-metal that gains electrons) ex. ClNaming Binary Ionic Compounds • Binary Ionic Compound – A compound that has 2 atoms, __________ and __________ – Cation is always __________ – Type I and Type II • Type I – The metal only forms __________ type of cation, ex. Li → Li+ • Type 2 – The metal can form _________________ cations, ex. Cu → Cu+ or Cu2+ • How can you tell if it is a Type I or Type II? – Type I: ________________, Al, Zn, Cd, and Ag, – _________________ are almost always Type II Rules for Naming Type I Ionic Compounds 1. Cation ( __________ ) is named before the anion (non-metal). 2. Cation (metal) is the name of the __________ . 3. Anion (non-metal) is the “ __________ ” of the element + ide 4. Combine the Cation and Anion Ex. NaCl 1. Cation is named before the anion. – we know that Na is first because it’s a cation 2. Cation is the name of the element. – Na is __________ 3. Anion is the “root” of the element + __________ – Cl is Chlorine, root is __________ = Chloride 4. Combine the Cation and Anion – ________________ is the name!! ****Notice: The formula doesn’t show the __________ of the individual ions. ****Ex. NaCl means Na+ is present with Cl****Ex. CaS means __________ is present with __________ • Let’s try some!! • CsF • CaBr2 • AlCl3 • ZnS • MgI2 • BaH2• KCl • Al2S3Binary Ionic Compounds Type II • Some metals can form more than __________. • This is what makes them Type II – Ex. Lead(Pb) => Pb2+ or __________ – Ex. Iron(Fe) => Fe2+ or __________ – ____________________ are almost always Type II • To name these we use the same naming method except we add a _______________ to the cation name. • The anion stays the same as before. EXTEMELY IMPORTANT!!! • The Roman numeral tells the __________ , not the # of ions present. • Ex. Fe3+ => iron(III) and Fe2+ => iron(II) • Ex. Pb2+ => lead(II) and Pb4+ => lead(IV) • The anion will be named the __________ as with Type I (using ide for the ending) • Anion is the “root” of the element + ide • Ex. O is Oxygen, root is Ox + ide = Oxide • Ex. Cl is Chlorine, root is Chlor + ide = Chloride • All together: • FeCl2 => __________ • PbO2 => __________ Lets take a closer look. • We are given FeCl2 • We know the overall charge has to be __________ • We know: • Cl is going to have a ____ charge. • there are 2 Cl • Cl2 has an overall charge of ______ • There is only one Fe atom • Therefore the one Fe has to have a charge of _____ Let’s try some!!! 1. CuCl 3. Fe2O 2. HgO Rules for naming Binary Covalent Compound: Prefix System 1. The first atom is the __________ . 2. The second atom is named like an __________ . 3. Add __________ to indicate # of atoms. Omit mono- prefix on the __________ element. Ex. PCl3 => phosphorus trichloride • These are the prefixes that are used to indicate the number of atoms in a compound. • __________ is never used for the first atom. • Binary Covalent Compound: • Example: __________ 1. The first atom is the element name. – I becomes iodine 2. The second atom is named like an anion. – O is Oxygen, root is __________ = oxide 3. Prefixes are given to each atom to tell the number of atoms present. – di = 2, hepta = 7 – so… di + iodine = diiodine – and… hepta + oxide = heptoxide – Answer: __________ • Let’s try some!!! 1. BF3 – 5. Al2S3 2. NO 6. SnBr4 3. N2O5 7. CS2 4. AsF3 8. CdS - Polyatomic Ions – Charged entities composed of ______ ____ bound together – Assigned __________ names – No system, must be __________ – (don’t worry you don’t have to, just know how to use it) – Ex. CN-, NH4+, NO3- , See pg 109 • Oxyanions – Some of these polyatomic ions are called __________ – They contain an atom and different #’s of oxygen atoms. – Ex. NO3-, ClO2-, PO43– These oxyanions, form in a __________. • The smaller # O, ends with _____. • The larger # O, ends with ____. • The smallest # O, begins with _____, meaning less than • The largest # O, begins with _____, meaning more than – All combined • __________ = hypo => ClO- = hypochlorite • Small = ite => ClO2- = ________ • Large = ate => ClO3- = __________ • _________ = per => ClO4- = perchlorate Now back to the naming complex compounds • Need to _________ common polyatomic ions • Now when you see NH4C2H3O2 you can break it down. – __________=> ammonium and C2H3O2+ => __________ – It’s name is ammonium acetate ****Notice that it’s separate parts have a __________ but together they are neutral. • Also like Type II, Binary Compounds, you may need ______________ • Ex. FeSO4 => iron(II) sulfate • Ex. Mn(OH)2 => manganese(II) hydroxide Lets practice some!! • Na2(SO)4• KH2PO4• Fe(NO3)3- Naming Acids!! Acids • Is a molecule with one or more ______ ions attached to an anion. • Translation: A molecule with _______ and non-metals • Ex. HCl, HCN, H2S Naming Acids • Two types: 1. Ones that __________ oxygen 2. Ones __________ oxygen Those __________ oxygen 1. Start with the _________of the anion. 2. Add the prefix __________ 3. Add the suffix __________ o Ex. hydrochloric acid o Ex. hydrofluoric acid Example: HCl 1. Start with the root of the anion. Cl is chlorine, root => __________ 2. Add the prefix ________ , hydro + chlore = hydrochlore 3. Add the suffix ______ . hydrochlore + ic = hydrochloreic 4. Answer: hydrochloreic acid A few exceptions • __________ => hydrocyanic acid • __________ => hydrosulfuric acid Those 1. 2. 3. • • • ____________ oxygen Start with the __________ of the anion. If the anion ends with an _______, Drop the ate and add ic If the anion ends with _______, Drop the ite and add ous Example: HC2H3O2 1. Start with the __________ of the anion. C2H3O2 is Acetate = > root is acet 2. If the anion ends with an ate, Drop the ate and add _________ 3. Answer: Acet + ic => __________ Example: HNO2 1. Start with the root of the __________. NO2 is nitrite = > root is nitr 2. If the anion ends with _______, Drop the ite and add ous 3. Answer: Nitr + ous => ________ A few exceptions Sulfur, H2SO4 => __________ acid and ________ => phosphoric acid Naming acids flow chart