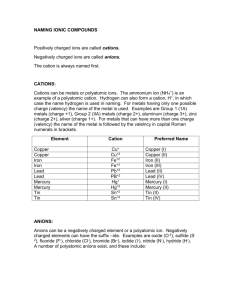
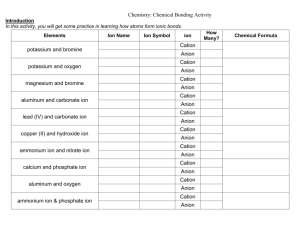
8.6 Acid base properties of salt solution Ions can also exhibit acidic
advertisement

8.6 Acid base properties of salt solution Ions can also exhibit acidic or basic properties. Such behavior implies that salt solutions can be acidic or basic Salts are strong electrolytes (completely dissociate) in aqueous solution Many ions react with water to generate H+ or OH- via rxn called Hydrolysis pH of salt solution can be predicted qualitatively considering salt cations and anions AN ANIONS ABILITY TO REACT WITH WATER In general, an anion X- in a solution can be considered a conjugate base of an acid Whether or not an anion reacts with water to produce hydroxide ions depends on the strength of anions conjugate acid To identify an acid and assess its strength we add proton to the anions formula If the acid HX is one of the seven strong acids HCl, H2SO4, HNO3, H3O+, HSO4-, H3PO4, HF – anion of these acids (conj. Base of an acid) has a negligible ability to abstract a proton from water and does not affect the pH of the solution. The presence of Cl- in a solution does not result in the production of any OH- and does not affect pH ( Cl- is a spectator ion in acid –base chemistry) If HX is not one of the strong acids then it is a weak acid and its conjugate base is a weak base and it reacts to a small extend with water to produce the weak acid and OH- ions X-(aq) + H2O(l) HX(aq) + OH-(aq) (OH- increases pH) Q: what effect will NO3- ions have on the solution, what effect would CO32-(aq) ions have on the solution? EX #1: Calculate the pH of a 0.35M solution of NaCHOO (Sodium methanoate) SALTS CONTAINING IONIZABLE PROTONS EX: HCO3-, HSO3-, these salts are amphiprotic- refers to the capacity of a substance to either add or loose a proton How they behave in water is determined by the relative magnitudes of Ka and Kb for the ion If Ka>Kb ion causes the solution to be acidic If Kb>Ka ion caused the solution to be basic COMBINED EFFECT OF CATION AND ANION SOLUTION To determined whether a salt forms and acidic, basic or neutral solution when dissolved in water we must consider the action of both cation and anion There are four possible combinations 1. Anion does not react with water, cation does not react with water – the solution Is neutral Ex: KNO3 2. Salt contains anion that reacts with water to produce OH- and cation that does not react with water, pH is basic Ex: Ba(CH3COOH)2 3. Salt contains cation that reacts with water to produce hydronium ions and anion that does not react with water pH is acidic Ex: NH4+ Ex: CH3NH3+ 4. Both anion and cation are capable to react with water to produce H3O+ and OH-, pH depends on relative abilities of the ions