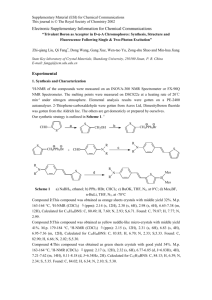
Synthesis and antibacterial activity of novel 11,12
advertisement

Synthesis and antibacterial activity of novel 11,12-cyclic carbonate azithromycin 4-O-carbamate derivatives Chenchen Ma, Zhaopeng Liu, Hualong Song, Rentao Jiang, Fawen He, Shutao Ma* Department of Medicinal Chemistry, School of Pharmaceutical Sciences, Shandong University, 44, West Culture Road, Jinan 250012, P. R. China Supplementary material Index: Synthetic Procedures S2 IR, 1H NMR and MS Spectra S3–S10 S1 2′- O-Acetyl-4-O-acylimidazolyl azithromycin 11,12-cyclic carbonate (6) Compound 6 was prepared from intermediate 2 according to the procedures17 reported by Ma S., et al. The crude 6 was purified by flash chromatography (dichloromethane–methanol, 20 : 1) to afford 6 (92.1%) as white solid: mp 117–120 °C; TLC Rf = 0.62 (dichloromethane–methanol, 10 : 1); ESI-MS m/z calculated for C45H74N4O15 911.1; found (M+H)+ 912.4. General methods for 11,12-cyclic carbonate azithromycin 4″-carbamate derivatives (7a–h) To a solution of 6 (1.33 g, 1.50 mmol) in DMF (15 ml) was added DBU (0.33 ml, 2.25 mmol) and corresponding amine (2.25 mmol). The resulting solution was stirred for 10 h at the room temperature. The reaction was quenched with water (30 ml) and the aqueous layer was extracted with ethyl acetate (3 × 15 ml). The combined organic layers were washed with brine (3 × 15 ml), and dried over anhydrous Na2SO4, filtered. The filtrate was concentrated in vacuo to afford a crude product. A solution of the above crude product in methanol (15 ml) was heated to 55 °C and stirred for 24 h at the same temperature. After concentrating the reaction solution in vacuo, the residue was purified by flash chromatography (dichloromethane–methanol, 20 : 1) to afford products 7a–h in yields ranging from 68.5% to 80.7%. 17. Ma, S., Jiao, B., Liu, Z., Wang, H., Xian, R., Zheng, M., Lou, H. Synthesis and antibacterial activity of 4,11-di-O-arylalkylcarbamoyl azithromycin derivatives that have activity against resistant strains. Bioorg. Med. Chem. Lett. 19, 1698–1701 (2009). S2 IR, 1H-NMR and MS Spectra of Compound 7a S3 IR, 1H-NMR and MS Spectra of Compound 7b S4 IR, 1H-NMR and MS Spectra of Compound 7c S5 IR, 1H-NMR and MS Spectra of Compound 7d S6 IR, 1H-NMR and MS Spectra of Compound 7e S7 IR, 1H-NMR and MS Spectra of Compound 7f S8 IR, 1H-NMR and MS Spectra of Compound 7g S9 IR, 1H-NMR and MS Spectra of Compound 7h S10