Maternal and neonatal influences on, and reproducibility of

In press Hypertension 2006
Maternal and neonatal influences on, and reproducibility of, neonatal aortic pulse wave velocity
Koudsi Abir
1
, Oldroyd John
1
, McElduff Patrick
2
, Banerjee Moulinath
1
,
Vyas A
1
, Cruickshank J Kennedy
1
1
Clinical Epidemiology & Cardiovascular Medicine Group, Division of Cardiovascular
& Endocrine Sciences, Manchester University and Royal Infirmary.
2
Evidence for Population Health Unit, University of Manchester, Medical School.
Correspondence to Professor JK Cruickshank on clinep@manchester.ac.uk
Prof JK Cruickshank
Division of Cardiovascular and Endocrine Sciences
Core Technology Facility (3rd Floor),
University of Manchester
46 Grafton Street, Manchester M13 9NT, UK.
Tel: 44-(0)161 275 1193;
Fax 44(0)161 275 1183
Key words: aortic pulse wave velocity, neonatal, pregnancy, maternal blood pressure, birthweight, reproducibility
2
Abstract:
Aortic pulse wave velocity (aPWV), a non-invasive measure of vascular stiffness, is an independent predictor of cardiovascular disease both before and in overt vascular disease.
Its characteristics in early life and its relationship to maternal factors have hardly been studied. To test the hypothesis that infant aortic pulse wave velocity was positively related to maternal anthropometry and blood pressure (BP) at 28 weeks gestation, after adjusting for neonatal anthropometry and BP, 148 babies born in Manchester UK were measured 1-3 days after birth. A high reproducibility of aPWV, assessed in 30 babies within 3 days of birth, was found with a mean difference between occasions of - 0.04,
95% CI – 0.08 to 0.16, m/s. Contrary to our hypothesis, a significant inverse relation was found between neonatal aPWV (mean 4.6 m/S) and maternal systolic BP (mean 108.9 mmHg) (r = -0.57, 95% CI –0.67 to –0.45) but not maternal height nor weight. Neonatal aPWV was positively correlated with birthlength, birthweight and SBP. In multiple regression, neonatal aPWV remained significantly inversely associated with maternal
SBP (adjusted β coefficient – 0.032, 95% CI -0.040 to – 0.024, P <0.001), after adjustment for maternal age, birth weight, length and neonatal BP (all independently and positively related to aPWV) and for gestational age, maternal weight and height
(unrelated).
These results suggest that infant aPWV may be a useful index of infant vascular status, is less disturbing to measure than infant BP, and is sensitive to the gestational environment marked by maternal BP.
[245 words]
3
Introduction:
Aortic pulse wave velocity (aPWV) in adults is a powerful predictor of all-cause and
CVD mortality and, as found in a least 4 outcomes studies [1-4], displaces systolic BP or pulse pressure as a predictive factor, perhaps because it is a direct index of vascular structure and function, further along the casual pathway of vascular pathology [1].
Primary prevention or early intervention in high blood pressure (BP) requires its early detection. It has become well established that high BP is frequently detectable in childhood as levels above the 90 th
centile for age [5]. However, little is known about the natural history of other arterial characteristics in childhood, such as distensibility and flow wave reflections.
Maternal factors during pregnancy, not least the mother’s own BP, have long been known to influence infant and later childhood vascular development and BP [6-10]. However, the maternal-infant BP relationship has been uncertain [11-14]. In part this may have been due to measurement errors, both random and systematic, from disturbance of the neonate by the observer until the recent availability of reliable semi-automatic methods.
It is clear that children of women with more extreme forms of hypertension in pregnancy, such as pre-eclampsia, have higher BP and risk of later vascular events than those of
‘control’ women [15;16] but across the range of usual BP in pregnancy, transmission of risk to the next generation is less clear [17]. Another approach may be to examine arterial distensibility in infancy.
To our knowledge there have been few studies of arterial distensibility in early life and most of them were done on small sample sizes [18-22]. Some methods have not been
4
validated in young age groups but direct measures of flow using Doppler methods offer a useful approach. To develop this area, we measured aPWV in a cohort of infants, testing the hypothesis that maternal BP would be directly and positively related to neonatal aPWV, independent of other maternal and infant factors.
Methods:
Participants:
The women were recruited around 20 weeks of pregnancy at the time of their second ultrasound scan. At 28 weeks gestation, a glucose tolerance test (GTT) was done and two measures of brachial BP were taken after the woman had been sitting for at least 5 minutes; the readings were taken 1 minute apart, using a validated Omron 705CP monitor with an appropriate sized cuff. The mean of the two readings was used for analysis.
Information on pre-pregnancy weight, birthweight, sociodemographic status and ethnicity, the participants’ family history of hypertension, weight and height were recorded .
We visited the mother and neonate within a day after delivery, when babies were measured for neonatal anthropometrics, BP and aPWV. Babies with vascular anomalies or with patent ductus arteriosus were not included. BP was measured with a Critikon
Dinamap machine, as in other neonatal studies [23]
,
a neonatal cuff and a rest period of
2 minutes in quiet surroundings. Infant BP was measured once before and once after aPWV and the mean of the two readings was used in the analysis. All newborn measurements were made by one paediatrician (AK) in the postnatal wards of St. Mary's
Maternity hospital, Manchester UK.
5
The protocol for measuring aPWV required the infant to lie in a supine position for 2-
5 minutes before testing. To examine the longest, least folded part of the aorta, we measured between the left subclavian artery at its origin from the aortic arch and the abdominal aorta just above the aortic bifurcation (on the skin around the umbilicus)
(Figure 1-A). An infra-red probe was placed in the supra-clavicular space or suprasternal notch aimed at the root of the left subclavian artery. A 4 MHz ultrasound transducer was placed over the abdominal aorta (Figure 1-B). The flow waves from these two aortic sites were simultaneously recorded. Two software systems for vascular compliance were used to measure aPWV: PULSE50.EXE (Data capture) and PULSE12.EXE (Analyser of time delay) [24;25]. The latter contains an algorithm [developed by Kontis & Gosling [26]] for detection of the onset of the systolic upstrokes (foot-to-foot) of the flow-velocity waveforms in the proximal and distal signals. High quality waveforms were captured (an average of 23, the minimum, to 30 waveforms); the distance between the sampling sites was measured with a tape. aPWV is calculated by dividing the distance travelled by the time differential between the two waveforms using the following formula: aPWV=
Probsep (m)/TT(s) , where TT is transit time between the two probes and Probsep is the distance between the sampling sites (Figure 1-C).
Reproducibility:
To test the reproducibility of aPWV measurements within subject measured by the same observer, 30 neonates had 2 aPWV recording sessions, at baseline and upto 1-3 days later. The Bland & Altman criteria were used to assess the level of agreement [27]: the mean difference between the two measurements for each individual (assessed using the 95% CI of the mean difference) should not be significantly different from zero. Also,
6
differences between the two measurements should be independent of their mean. We also estimated within subject coefficient of variation [28].
Data are presented as proportions and means for the 2 groups, defined by the median of maternal Systolic BP (SBP) at 28 weeks gestation. Comparison of proportions between groups was tested using Chi-square test, t-tests and analysis of variance, with 95% confidence intervals where appropriate.
The univariate or unadjusted relationship between infant aPWV and maternal SBP was examined using simple linear regression. To adjust for potential confounding factors and to identify the independent predictors of aPWV at birth, we fitted a multiple linear model using backward stepwise regression. A forward stepwise model gave very similar results (data not shown). Other maternal factors included were sociodemographic status, ethnicity, age, 2-hour plasma glucose values post-GTT, pre-pregnancy height and weight, weight gain at 28 weeks gestation; infant factors were birth size (as either birthweight or birthlength), gestational age, gender, SBP and heart rate. Analyses were done with the statistical software packages of intercooled STATA version 8.2 and SPSS version 11.5.
Results:
Reproducibility study:
The mean difference in aPWV between the two occasions was -0.04 m/s (95% CI -
0.08 to + 0.16 m/s - note including zero), indicating not only a minimal absolute difference between paired readings, but also that there was no evidence of a systematic difference between the first and second measurement. The difference in aPWV between the two occasions was plotted against the mean of both measures, and showed that the
7
variation in differences was relatively constant with increasing values of aPWV (Figure
2). The within subject coefficient of variation was 0.05 or 5%.
Pulse wave velocity study:
148 women with a mean age of 31.9 (95% CI 30.9 to 32.8) years were included.
Fifty four percent (82) classified themselves as (and were also by origin of 3 out of 4 grandparents) White European and 28% (35) were similarly classified as Pakistani.
The mean gestational age of the babies was 39.1 (38.8 to 39.5) weeks, birth weight
3418 (3342 to 3495) grams, mean birth length 50.5 cm (50.1 to 50.9 cm) and aPWV was
4.6 (4.53 to 4.73) m/s.
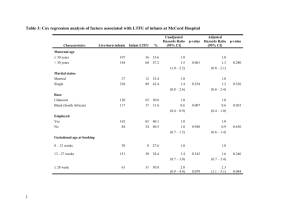
Table 1 shows maternal and infant characteristics between groups defined by median maternal SBP at 28 weeks (108 mmHg). The mean aPWV was higher in babies whose mothers’ SBP at 28 weeks was < 108 mmHg than babies whose mothers’ SBP at
28 weeks was
108 mmHg (P < 0.001). Reported pre-pregnancy weights of women in the upper half of the BP distribution were 3 kg heavier on average than those in the lower half, which meant their mean pre-pregnancy BMIs were 1.3 kg/m 2 higher. Weight gained at 28 weeks was similar. There were no other differences in maternal or neonatal characteristics, except as defined by maternal BP. Infant BP and anthropometry were also similar between infant groups defined by maternal BP (Table 1).
Maternal SBP at 28 weeks gestation showed a strong inverse relationship with aPWV (r = -0.57 ( 95% CI –0.67 to –0.45)) (Figure 3). Maternal SBP was also inversely associated with infant birth weight (r = -0.18, -0.33 to –0.02) but there was no statistically significant association between maternal and infant SBP (r = -0.11, -0.26 to + 0.06).
8
Maternal weight and height at 28 weeks were not associated with the infants SBP, DBP, heart rate or aPWV.
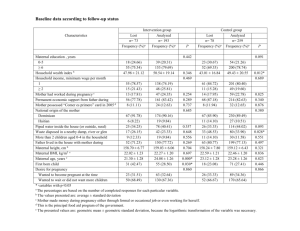
In simple linear regression models, maternal BP and age were statistically significant predictors of the babies’ aPWV (Table 2). Birth weight, birth length, neonatal
S & D BP and heart rate but not pulse pressure were also individually significantly associated with aPWV. There were no differences found by gender or ethnic groups, nor were sociodemographic nor family history variables significant.Only maternal SBP, maternal age, infant length and infant SBP were statistically significant predictors of aPWV in a multiple regression model selected using backward stepwise regression.
Forward stepwise regression models gave the same results. The four predictors in the final model (Table 2, right hand column) explained 43% of the total variation in aPWV.
When associations of neonatal SBP were tested, even after excluding aPWV, none of the maternal or infant variables were significantly related, except gender (males had significantly higher SBP than females P = 0.03) with maternal SBP marginally so (P =
0.064). Infant size, measured as either birthlength or birthweight, was not associated with infant SBP.
Discussion:
To the best of our knowledge this report is the first to identify the relationship of maternal factors with neonatal aPWV. We found a strong inverse relation between maternal SBP at 28 weeks and neonatal aPWV, and good reproducibility of neonatal aPWV in the first days of postnatal life.
9
The reproducibility results are similar to those we obtained in adults whose mean age was around 60 years [26]. That the aPWV method does give such reproducibility at two widely separated times of life may be due to capturing the 23-30 cardiac cycles that generate the mean aPWV result. This differs from most BP data, including those here, which are based on means of only 2 or 3 systolic and diastolic cycles and are inevitably less precise estimates of an average BP level. Despite the imprecise characterisation of maternal BP at 28 weeks gestation, the inverse relation we found with neonatal aPWV was strong (Figure 3) and robust, being independent of other potential and neonatal confounders such as anthropometry (Table 2). Further, this relationship was found in a sample of healthy, term neonates, none of whose mothers was on anti-hypertensive treatment. The distribution of maternal SBPs was also right across the usual low-medium range for pregnancy, with very few high values (see the horizontal axis of Figure 3).
The average of 4.6 m/s for aPWV at birth is close to that reported in slightly older infants and young children [18]. The finding of the inverse relation between BP and neonatal aPWV is the opposite of our original hypothesis. There are few reports on PWV in infancy/early childhood available with which to compare our data [18-22], and none with the relatively large number of neonates we have studied.
The mechanisms by which maternal blood flow modulates neonatal arterial growth are not known. The development of the aortic media involves the formation of alternating layers of smooth muscle cells and elastic laminae [29]. The number of elastic lamellae is greatest in the proximal part of the aorta. They begin to develop early in fetal life, and rates of elastin synthesis in blood vessels increase to a maximum in the perinatal period; thereafter these rates fall rapidly [30]. There seems to be a critical period during
10
development of the aorta and failure to synthesise adequate amounts of elastin during this period is apparently impossible to replace later. In lambs, between 120 days of gestation and 21 days after birth, thoracic aortic media cross-sectional area increased by 144% whereas this index for abdominal aorta increased by only 69%. These differences in growth rates were greater postpartum [31]. How these characteristics of thoracic and abdominal aorta are reflected by aPWV is not known. At this age, the thoracic aorta grows more quickly than the abdominal aorta; it is possible that infants of women with higher maternal BP in pregnancy have adapted with slightly greater thoracic aortic medial cross-sectional area and therefore lower aPWV. Conversely, in infants of mothers with lower BP (and placental blood flow) in pregnancy, there may be some degree of compensatory vasoconstriction, as happens to close the patent ductus. Animal data suggest enhanced vasoconstriction is a feature of vascular smooth muscle during pregnancy [32]. It is also of interest that Steer et al reported a ‘u’-shaped relationship between perinatal outcome and maternal diastolic BP [33].
An intriguing issue, answerable by continued longitudinal study through early childhood and later life, is which infants go on to develop stiffer, less distensible, aorta later in childhood and adult life. Results from work over 20 years ago support this idea
[18]. Laogun and Gosling’s work on schoolchildren showed that aPWV (again measured from the arch to just above the bifurcation to minimize reflection artefact) fell slowly in early childhood but then decreased markedly around the ages of 10-12 years in both boys and girls, perhaps related to puberty; hence its inverse, aortic compliance or distensibility, increased to its maximum at these ages. Thereafter from age 14 years or so compliance declined inexorably over the lifespan (ie: aPWV increased). It is likely that several of the
11
factors we have measured here, and then others relating to postnatal growth, influence this rise in compliance or fall in aPWV in later childhood, and then its ongoing decline.
Our hypothesis would be that the children whose mothers were in the upper parts of the maternal BP distribution will begin to show by that time, increasing stiffness in their aorta, in part independent of their BP.
It is possible that the changes in cardiac physiology around birth and the closing of the patent ductus arteriosus (PDA) could be important influences on aPWV. We only included babies in whom clinically the PDA was no longer detectable. Other perinatal haemodynamic changes including rising pulmonary pressures and their consequences could not be assessed but we aimed to minimise these effects by measuring most babies
24 hours or more after birth. Undetected abnormal flows seem unlikely to account for the consistent maternal BP/aPWV relationship we found.
Our results of positive correlations between aPWV at birth and both birthweight and birthlength contrast with the finding of an inverse relation between birthweight and PWV in later life [34;35], attenuated after adjusting for current height [35]. In young adulthood, the inverse correlation was not consistent [36;37] and in children and adolescents aortic stiffness in particular has shown little relationship with birthweight [38]. However, the positive relationship reported by Oren et al was in agreement with our findings [37].
Differences with respect to age, methods used to assess arterial stiffness, the arterial segments measured, and adjustment for confounders make the studies that investigated the relationship difficult to compare. However, note our ‘adaptive’ hypothesis above, by which arteries may later ‘stiffen’ inexorably after peak distensibility has been reached around adolescence. We measured aPWV using the arch-abdominal segment as the wave
12
travels between these two sites in one direction and the effect of reflection from the peripheral arterial tree in the aorta is minimal, hence interfering in the flow wave form harmonics much less. Also, to reduce the influence of body contours on the distance measure (between the two sites) the tape measure was held above the surface of the body, parallel to the plane of the examination table. The length value (to the nearest 0.1 cm) was taken in duplicate straight after capturing the signals and the mean was used for aPWV calculation. Movements of the subjects could cause problems in getting consistent high quality waveforms; however, the high heart rate in infancy made the procedure easier by allowing rapid recapture once the neonate settled.
SBP at birth was on an average of 70.7 mmHg for the whole group of babies, which is consistent with that reported for term infants (70 mmHg) [39]. As early as one day of life, Miller et al showed a significant relation between a neonate’s BP and high maternal
BP during pregnancy [40]. Similar significant correlations have been found during the following two days of the child life [6;41;42] . The maternal-infant SBP relation was of borderline significance in our study whereas maternal SBP-neonatal aPWV relation was stronger and inverse. Adjusting for confounding did not alter the later correlation. It thus seems that neonatal aPWV seems to be a more precise indicator of maternal environment during pregnancy than neonatal SBP.
In conclusion, these results suggest that measurement of neonatal, aPWV was reproducible, may be closely but inversely related to maternal BP around 28 weeks gestation, and more so than neonatal BP. aPWV may also therefore be a useful index of arterial structure and function at this age, and by implication, in later infancy and
13
childhood, as in adult life. Assessing the effects of differing maternal conditions during pregnancy on the infant’s vasculature may be a useful area of further work.
Acknowledgment:
We thank Professor S Greenwald for his help in setting up some of the initial equipment.
14
Reference lists:
(1) Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation.
2002;
106:2085-2090.
(2) Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent
S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension.
2002; 39:10-15.
(3) Laurent S, Katsahian S, Fassot C, Tropeano A, Gautier I, Laloux B, Boutouyrie P.
Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke.
2003; 34:1203-1206.
(4) Blacher J, Safar M, Guerin A, Pannier B, Marchais S, London G. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int.
2003;
63:1852-1860.
(5) The Fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics.
2004; 114:555-576.
(6) Zinner SH, Lee YH, Rosner B, Oh W, Kass EH. Factors affecting blood pressures in newborn infants. Hypertension.
1980; 2:99-101.
15
(7) Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL Preeclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol.
1991; 98:1009-1014.
(8) Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH.
Initiation of hypertension in utero and its amplification throughout life. BMJ.
1993; 306:24-27.
(9) Himmelmann A, Svensson A, Hansson L. Relation of maternal blood pressure during pregnancy to birth weight and blood pressure in children. The
Hypertension in Pregnancy Offspring Study. J Intern Med.
1994; 235:347-352.
(10) Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO.
Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol.
2003; 101:529-533.
(11) Ibsen KK, Gronbaek M. Familial aggregation of blood-pressure in newly born infants and their mothers. Acta Paediatr Scand.
1980; 69:109-111.
(12) Mausner JS, Hiner LB, Hediger ML, Gabrielson MO, Levison SP. Blood pressure of infants of hypertensive mothers: a two-year follow-up. Int J Pediatr Nephrol.
1983; 4:255-261.
16
(13) Hashimoto N, Kawasaki T, Kikuchi T, Takahashi H, Uchiyama M. The relationship between the intrauterine environment and blood pressure in 3-yearold Japanese children. Acta Paediatr.
1996; 85:132-138.
(14) Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman
KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J
Pediatr.
2004; 144:240-245.
(15) Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Preeclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol.
1991; 98:1009-1014.
(16) Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO.
Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol.
2003; 101:529-533.
(17) Churchill D, Perry IJ, Beevers DG. Ambulatory blood pressure in pregnancy and fetal growth. Lancet.
1997; 349:7-10.
(18) Laogun AA, Gosling RG. In vivo arterial compliance in man. Clin Phys Physiol
Meas.
1982; 3:201-212.
17
(19) Hu J, Wallensteen M, Gennser G. Increased stiffness of the aorta in children and adolescents with insulin-dependent diabetes mellitus. Ultrasound Med Biol.
1996;
22:537-543.
(20) Ramos E, Perez-Quintero J, Encimas S, Olivan J, Gonzalez-Hachero J, Perez-
Cano R. Carotid-femoral pulse wave velocity in children and adolescents from 2-
18 years. Hypertension.
1999; 33:1307.
(21) Hsieh KS, Chen PL, Fu SE. A simple, noninvasive method to investigate vascular characteristics in children. Angiology.
1996; 47:361-367.
(22) Gardiner HM, Taylor MJ, Karatza A, Vanderheyden T, Huber A, Greenwald SE,
Fisk NM, Hecher K. Twin-twin transfusion syndrome: the influence of intrauterine laser photocoagulation on arterial distensibility in childhood.
Circulation.
2003; 107:1906-1911.
(23) Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman
KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J
Pediatr.
2004; 144:240-245.
(24) Greenwald S, Denyer H, Sobeh MS. Noninvasive measurement of vascular compliance by a photoplesythmographic technique. SPIE (The International
Society for Optical Engineering).
1997; 2970:89-97.
18
(25) Loukogeorgakis S, Dawson R, Phillips N, Martyn CN, Greenwald SE. Validation of a device to measure arterial pulse wave velocity by a photoplethysmographic method. Physiol Meas.
2002; 23:581-596.
(26) Wright JS, Cruickshank JK, Kontis S, Dore C, Gosling RG. Aortic compliance measured by non-invasive Doppler ultrasound: description of a method and its reproducibility. Clin Sci (Lond).
1990; 78:463-468.
(27) Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet.
1986; 1:307-310.
(28) Bland M. An introduction to medical statistics. 3rd ed. Oxford: Oxford University
Press, 2000.
(29) Davis EC. Elastic lamina growth in the developing mouse aorta. J Histochem
Cytochem.
1995; 43:1115-1123.
(30) Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet.
1997; 350:953-955.
(31) Langille BL, Brownlee RD, Adamson SL. Perinatal aortic growth in lambs: relation to blood flow changes at birth. Am J Physiol.
1990; 259:H1247-H1253.
19
(32) Dieye AM, Gairard A. Endothelium and aortic contraction to endothelin-1 in the pregnant rat. Can J Physiol Pharmacol 2000; 78: 372-7.
(33) Steer PJ, MP, Kold-Jensen T , Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study.
BMJ 2004; 329: 1312-16.
(34) Martyn CN, Barker DJ, Jespersen S, Greenwald S, Osmond C, Berry C. Growth in utero, adult blood pressure, and arterial compliance. Br Heart J.
1995; 73:116-121.
(35) te Velde SJ, Ferreira I, Twisk JW, Stehouwer CD, van Mechelen W, Kemper HC.
Birthweight and arterial stiffness and blood pressure in adulthood--results from the Amsterdam Growth and Health Longitudinal Study. Int J Epidemiol.
2004;
33:154-161.
(36) Montgomery AA, Ben Shlomo Y, McCarthy A, Davies D, Elwood P, Smith GD.
Birth size and arterial compliance in young adults. Lancet.
2000; 355:2136-2137.
(37) Oren A, Vos LE, Bos WJ, Safar ME, Uiterwaal CS, Gorissen WH, Grobbee DE,
Bots ML. Gestational age and birth weight in relation to aortic stiffness in healthy young adults: two separate mechanisms? Am J Hypertens.
2003; 16:76-79.
(38) Lurbe E, Torro MI, Carvajal E, Alvarez V, Redon J. Birth weight impacts on wave reflections in children and adolescents. Hypertension.
2003; 41:646-650.
20
(39) Bartosh SM, Aronson AJ. Childhood hypertension. An update on etiology, diagnosis, and treatment. Pediatr Clin North Am.
1999; 46:235-252.
(40) Miller FC, Read JA, Cabal L, Siassi B. Heart rate and blood pressure in infants of pre-eclamptic mothers during the first hour of life. Crit Care Med.
1983; 11:532-
535.
(41) Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Preeclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol.
1991; 98:1009-1014.
(42) Zinner SH, Rosner B, Oh W, Kass EH. Significance of blood pressure in infancy.
Familial aggregation and predictive effect on later blood pressure. Hypertension.
1985; 7:411-416.
21
Table 1: Characteristics of 148 participating mothers and their newborns by the median of maternal SBP at 28 weeks gestation
Maternal SBP < 108
mmHg (73)
Maternal SBP
108
mmHg (75)
Ethnicity
White European
Pakistani
Other ethnic groups
38 (52.1)
18 (24.7)
17 (23.3)
No. Subjects (%)
44 (58.7)
17 (22.7)
14 (18.7)
Maternal Factors
Age (years)
Height (cm)
Pre-pregnancy weight (kg)
Pre-pregnancy BMI (kg/m 2 )
Weight at 28 weeks gestation
(kg)
Weight gain to 28 weeks (kg)
DBP at 28 weeks (mmHg)
Pulse pressure at 28 weeks
(mmHg)
Heart rate at 28 weeks gestation (beats/m)
2-hours GTT (mmol/l)
Infant factors at birth
Gestational age (weeks)
Birthweight (g)
Birthlength (cm)
SBP (mmHg)
DBP (mmHg)
Pulse pressure
Heart rate (beats/min) aPWV (m/s)
31.9 (6.4)
160.9 (6.6)
63.2 (12.6)
24.4 (4.7)
75.8 (15.2)
12.0 (5.6)
66.8 (6.9)
34.0 (7.6)
85.6 (8.7)
6.12 (1.55)
39.1 (1.8)
3439 (461)
50.6 (2.5)
71.0 (4.3)
37.5 (6.0)
33.5 (3.7)
141 (6)
4.87 (0.55)
Mean (SD)
31.8 (5.5)
161 (7.6)
66.3 (13.9)
25.7 (5.4)
79.8 (14.1)
12.7 (5.4)
74.3 (7.7)*
41.2 (5.7)*
86.7 (11.4)
6.19 (1.51)
39.2 (2)
3404 (491)
50.4 (2.6)
70.5 (4)
37.1 (5.8)
33.4 (2.9)
141.4 (5.2)
4.41 (0.59)*
22
* P value < 0.001 for comparison of the two groups defined by Maternal SBP
Table 2: Factors associated with newborn aPWV (m/s) from a linear regression
Maternal factors
Ethnicity
White European
Pakistani
Other ethnic groups
Pre-pregnancy height (10 cm)
Pre-pregnancy weight (10 kg)
Pre-pregnancy BMI (1kg/m 2 )
Maternal factors at 28 weeks gestation
Age (10 years)
Systolic blood pressure (10 mmHg)
Diastolic blood pressure(10 mmHg)
Pulse pressure(10 mmHg)
Heart rate(10 beats/minute)
2-hour pl glucose (1 mmol/l)
Weight gain to 28 weeks (1 kg)
Weight (10 kg)
Infant factors at birth
Gestational age (1 week)
Birth weight (100 grams)
Birth length (10 cm)
Systolic blood pressure (10 mmHg)
Diabolic blood pressure (10 mmHg)
Pulse pressure (10 mmHg)
Coefficient (95% confidence interval)
Unadjusted
(Univariate)
0.02 (-0.22 to 0.27)
-0.00 (-0.26 to 0.25)
0.03 (-0.13 to 0.18)
-0.04 (-0.12 to 0.04)
-0.01 (-0.04 to 0.01)
0.16 (-0.01 to 0.33)
- 0.36 (-0.44 to –0.27)
-0.37 (-0.48 to –0.28)
-0.15 (-0.28 to –0.02)
-0.13 (-0.23 to –0.03)
-0.06 (-0.12 to +0.00)
-0.02 (-0.04 to +0.00)
-0.06 (-0.13 to 0.01)
-0.02 (-0.08 to 0.03)
0.04 (0.02 to 0.06)
0.85 (0.48 to 1.22)
0.37 (0.14 to 0.60)
0.26 (0.09 to 0.42)
-0.23 (-0.53 to 0.06)
R 2
0.0%
0.1%
0.7%
1.4%
2.4%
32.0%
25.2%
3.4%
4.6%
2.3%
2.3%
2.0%
0.6%
11.3%
12.4%
6.2%
6.1%
1.6%
Adjusted
(Multivariate)
0.13 (0.000 to 0.06) †
-0.32 (-0.40 to -0.24) ‡
0.59 (0.28 to 0.90) ‡
0.24 (0.05 to 0.42) §
Heart rate (10 beat/minute) 0.23 (0.05 to 0.40) 4.2%
† p = 0.04, ‡ p = 0.01, § p < 0.001, after adjusting for maternal age, ethnicity, height, weight, age, SBP, HR,
2-hour pl glucose, weight gain at 28 weeks gestation, gestational age, and also for infant birth weight, birth length, SBP, DBP and heart rate at birth.
The coefficients in these models represent the change in the predicted value of aPWV that is associated with each unit change (as specified) in the predictor variable of interest with these units given in the brackets after the variable name.
Note: not all variables could be included in the one model because they would be co-linear combinations of each other such as pulse pressure with diastolic and systolic blood pressure and weight change with pre-pregnancy and pregnancy weight.
23
Figure legends
Figure1: Diagram of A): the arterial segment measured *;B) use of the probes on an infant (with signed permission from the mother); and C) Waveforms captured simultaneously from the aortic arch and abdominal aorta and the time delay between the recordings. aPWV = aortic pulse wave velocity.
* http://www.qmw.ac.uk/~ugha096/sbs025/Lecture_19-Heart_Development_6.pdf
Figure 2: Reproducibility shown as neonatal aPWV differences between values 1-3 days later and at baseline plotted against the mean of aPWV on the two occasions. aPWV = aortic pulse wave velocity. Means for the baseline and the second (repeated) measurement were 4.56 and 4.61m/s respectively, and the difference between these two means was -0.04 (95% CI -0.08 to +0.11) m/s.
Figure 3: A plot of maternal SBP at 28 weeks gestation in 148 participants against infant aPWV at birth.
The mean regression line and the individual 95% confidence interval lines are shown.
The regression equation is: Neonatal aPWV = 8.50-0.04 * maternal SBP at 28 weeks gestation, R-Square = 0.32. aPWV: aortic pulse wave velocity, and SBP = Systolic Blood pressure
24
A
B
C
Figure 1
25
Figure 2
26
Figure 3
27