Pg 197 # 41-42, 48-49
advertisement
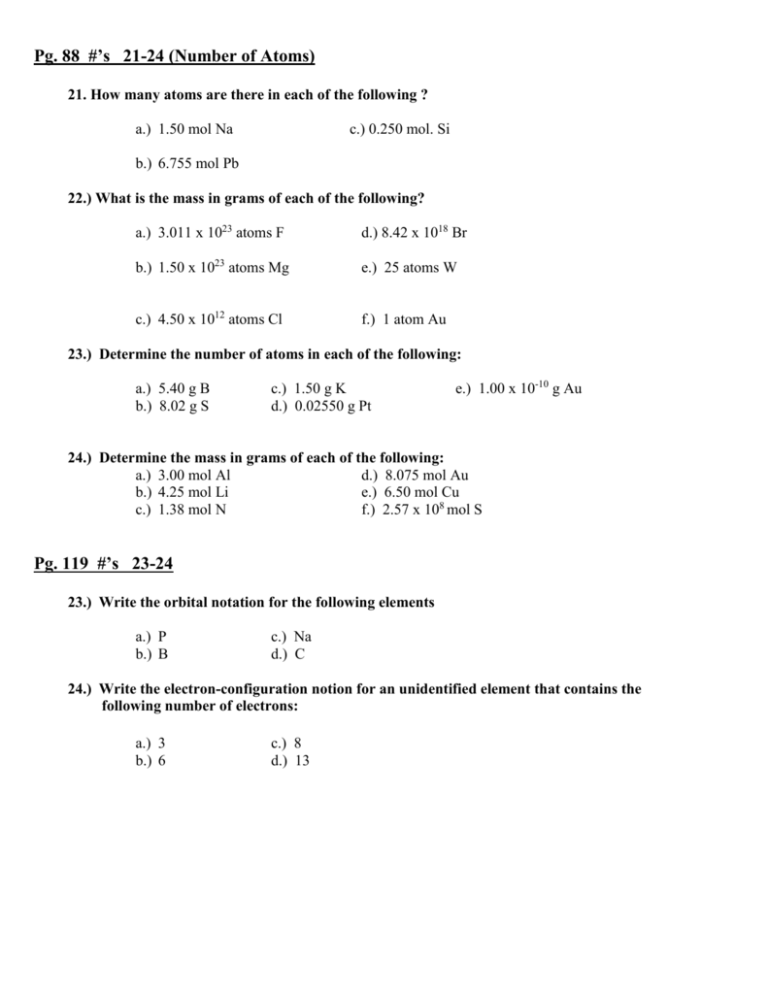
Pg. 88 #’s 21-24 (Number of Atoms) 21. How many atoms are there in each of the following ? a.) 1.50 mol Na c.) 0.250 mol. Si b.) 6.755 mol Pb 22.) What is the mass in grams of each of the following? a.) 3.011 x 1023 atoms F d.) 8.42 x 1018 Br b.) 1.50 x 1023 atoms Mg e.) 25 atoms W c.) 4.50 x 1012 atoms Cl f.) 1 atom Au 23.) Determine the number of atoms in each of the following: a.) 5.40 g B b.) 8.02 g S c.) 1.50 g K d.) 0.02550 g Pt e.) 1.00 x 10-10 g Au 24.) Determine the mass in grams of each of the following: a.) 3.00 mol Al d.) 8.075 mol Au b.) 4.25 mol Li e.) 6.50 mol Cu c.) 1.38 mol N f.) 2.57 x 108 mol S Pg. 119 #’s 23-24 23.) Write the orbital notation for the following elements a.) P b.) B c.) Na d.) C 24.) Write the electron-configuration notion for an unidentified element that contains the following number of electrons: a.) 3 b.) 6 c.) 8 d.) 13 Pg. 158 # 46 46.) For each element listed below, determine the charge of the ion most likely to be formed and the identity of the noble gas whose electron configuration is thus achieved. a.) b.) c.) d.) Li Rb O F e.) f.) g.) h.) Mg Al P S i.) Br j.) Ba Pg 197 #’s 41-42, 48-49 41.) Draw a Lewis structures for each of the following molecules. Show resonance structures, if they exist. a.) O2 b.) N2 c.) CO d.) SO2 42.) Draw a Lewis structures for each of the following polyatomic ions. Show resonance structures, if they exist. a.) OH- c.) BrO3- b.) H3C2O248.) Draw a Lewis structures for each of the following molecules, and then use VSEPR theory to predict molecular geometry. a.) SCl2 b.) PI3 c.) Cl2O d.) NH2Cl e.) SiCl3Br f.) ONCl 49.) Draw a Lewis structures for each of the following polyatomic ions, and then use VSEPR theory to determine the geometry of each. a.) NO3 b.) NH4+ c.) SO42 d.) ClO2 – Pg. 235-237 #’s 10-11, 27- 28,42- 44 10.) Name the following binary molecular compounds according to the prefix system. a.) CO2 b.) CCl2 c.) PCl5 d.) SeF6 e.)As2O5 11.) Write formulas for each of the following binary molecular compounds. a.) carbon tetrabromide b.) Silicon dioxide c.) tetraphosphorus decoxide d.) diarsenic trisulfide 27.) Assign oxidation numbers to each atom in the following compounds. a) HI b) PBr3 c) GeS2 d) KH e) As2O5 f) H3PO4 28.) Assign oxidation numbers to each atom in the following ions. a) NO3 b) ClO4 - c) PO4 3 d) Cr2O7 2 - e) CO3 2- 42.) Write the chemical formulas for the following compounds. a.) aluminum fluoride b.) magnesium oxide c.) vanadium (V) oxide d.) cobalt( II) sulfide e.) strontium bromide f.) sulfur trioxide 43.) How many atoms of each element are contained in a single formula unite of iron( III ) formate, Fe(CHO2)3 •H2O? What percentage by mass of the compound is water? 44.) Name each of the following acids, and assign oxidation numbers to the atoms in each. a.) HNO2 c.)H2CO3 b.) H2SO3 d.) HI Pg. 271 #’s 27-29 27.) complete and balance the equation for each of the following single-replacement reactions. a.) Zn + Pb( NO3)2 ___________________ b.) Al + Hg(CH3COO)2 ____________________________ c.) Al + NiSO4 _________________________________ d.) Na + H2O _____________________ 28.) complete and balance the equation for the following double- replacement reaction: a.) AgNO3(aq ) + NaCl (aq ) b.) Mg( NO3)2 (aq ) + KOH(aq ) c.) LiOH (aq ) + Fe(NO3)3 (aq ) ___________________________ __________________________ _________________ 29.) complete and balance the equation for the following combustion reactions: a.) CH4 + O2 ________________ b.) C3H6 + O2 ________________ c.) C5H12 + O2 ________________ Pg. 296 #’s 10-15 10.) Hydrogen and oxygen react under a specific set of conditions to produce water according to the following: 2H2 (g) + O2 (g) 2H2O. a.) How many moles of hydrogen would be required to produce 5.0 mol. of water? b.) How many moles of oxygen would be required? 11.) a.) If 4.50 mol. of ethane, C2H6, undergo combustion according to the unbalanced equation C2H6 + O2 CO2 + H2O, how many moles of oxygen are required? b.) How many moles of each product are formed? 12.) Sulfuric acid reacts with sodium hydroxide according to the following: H2So4 + NaOH Na2SO4 + H2O. a.) Balance the equation for this reaction. b.) What mass of H2SO4 would be required to react with 0.75 mol. Of NaOH? c.) What mass of each product is formed by this reaction? 13.) Sodium chloride is produced from its elements through a synthesis reaction. What mass of each reactant would be required to produce 25.0 mol of sodium chloride? 14.) Copper reacts with silver nitrate through single replacement. a.) If 2.25g of silver are produced from the reaction, how many moles of copper( II ) nitrate are also produced? b.) How many moles of each reactant are required in this reaction? 15.) Iron is generally produced from iron ore through the following reaction in a blast furnace: Fe2O3 (s) + CO (g) Fe(s) + CO2 (g) a.) If 4.00 kg of Fe2O3 are available to react, how many moles of CO are needed? b.) How many moles of each product are formed? Pg. 328 #’s 31-36 ( Combined Gas Law) 31.) A sample of gas at 47o C and 1.03 atm occupies a volume of 2.20 L. What volume would this gas occupy at 107o C and 0.789 atm? 32.) A 350. mL air sample collected at 35o C has a pressure of 550. torr. What pressure will the air exert if it allowed to expand to 425 mL at 57o C 33.) A gas measures 1.75 L at -23o C and 150. kpa. At what temperature would the gas occupy 1.30 L at 210. kpa? 34.) A sample of oxygen at 40.oC occupies a volume of 820. mL. If this sample later occupies 1250 mL at 60.oC and 1.40 atm, what was its original pressure? 35.) A gas at 7.75 x104 Pa and 17o C occupies a volume of 850.cm3. At what temperature, in degrees Celsius, would the gas occupy 720.cm3 at 8.10 x 104 Pa? 36.) A meteorological balloon contains 250. L of He at 22o C and 740.mm Hg. If the volume of the ballon can vary according to external conditions, what volume would it occupy at an altitude at which the temperature is -52o C the pressure is 0.750 atm? Pg. 479 # 36 36.) Name or give the molecular formula for each of the following acids: a.) HF b.) acetic acid c.) phosphorous acid d.) HClO4 e.) H3PO4 f.) hydrobromic acid g.) HClO h.) H2CO3 i.) sulfuric acid Pg. 505. #’s 15-18 ( pH calculations) 15.) Calculate the [H3O+] and [OH-] for each of the following. a.) 0.03 M HCl c.) 5 x 10-3 b.) 1 x 10-4 M NaOH d.) 0.01 M Ca(OH)2 16.) Determine the pH of each of the following solutions. a.) 1.0 x 10-2 M HCl c.) 1.0 x 10-5 M HI b.) 1.0 x 10-3 M HNO d.) 1.0 x 10-4 M HBr 17.) Given the following [OH-] values, determine the pH of each solution. a.) 1.0 x 10-6 M b.) 1.0 x 10-9 M c.) 1.0 x 10-2 M d.) 1.0 x 10-7 M 18.) Determine the pH of each solution. a.) 1.0 x 10-2 M NaOH b.) 1.0 x 10-3 M KOH c.) 1.0 x 10-4 M LiOH










