Lesson 31 questions – Solids Liquids Gases - science
advertisement
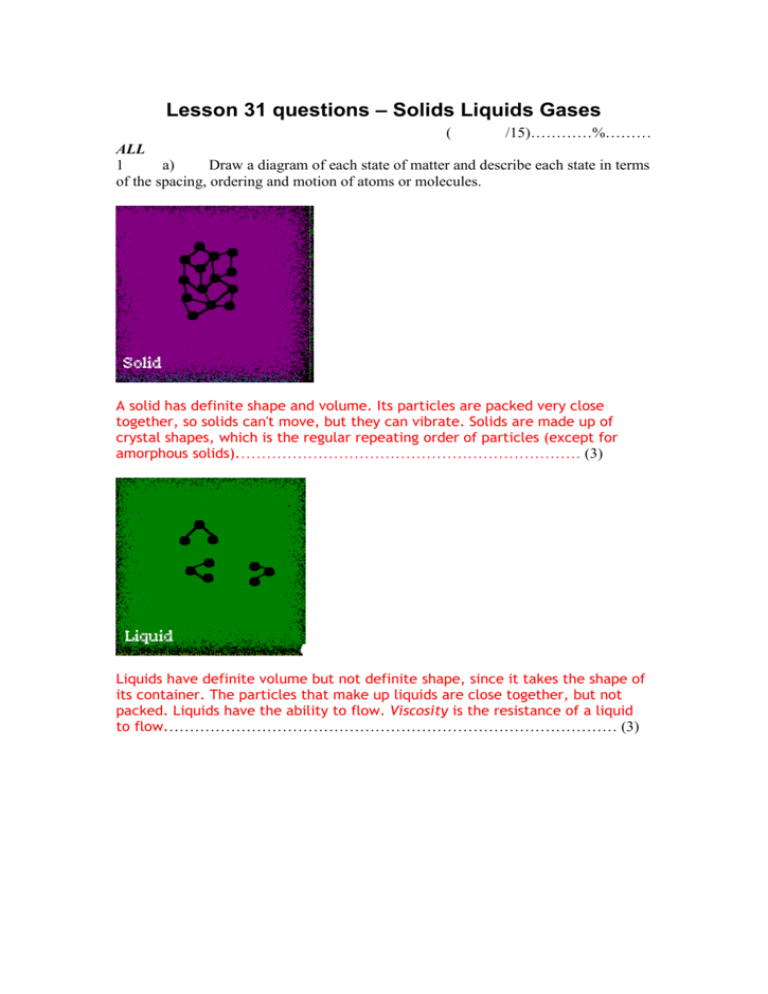
Lesson 31 questions – Solids Liquids Gases ( /15)…………%……… ALL 1 a) Draw a diagram of each state of matter and describe each state in terms of the spacing, ordering and motion of atoms or molecules. A solid has definite shape and volume. Its particles are packed very close together, so solids can't move, but they can vibrate. Solids are made up of crystal shapes, which is the regular repeating order of particles (except for amorphous solids).………………………………………………………… (3) Liquids have definite volume but not definite shape, since it takes the shape of its container. The particles that make up liquids are close together, but not packed. Liquids have the ability to flow. Viscosity is the resistance of a liquid to flow.…………………………………………………………………………… (3) Gases have neither a definite shape or volume. Their particles are spaced far apart and they are able to move freely.……………………………………………………………………………… (3) SOME Extension The particles of plasma are very far apart. It also has very high energy, which makes it quite dangerous. Although it is rare on Earth, it is the most common phase in the universe. The sun and stars are made of plasma. b) Describe what is meant by a Kinetic Molecular Theory for Solids Liquids and Gases. Particles are in constant motion. In solids the particles are close together and have limited motion. In a liquid some of the attraction between particles is overcome which allows the particles more freedom of movement. In a gas particles attraction between particles is minimized and the particles move freely throughout the container. …………………………………………………………………………………… (2) SOME 2 A Student asks on a Physics blog “If all matter is either solid, liquid, gas or plasma, what state of matter is a single atom or molecule?” What would you write in answers to this question? ……There are more states of matter than just Solid Liquid and Gas. (1) Solid, amorphous solid, liquid, gas, plasma, super-fluid, supersolid, degenerate matter, neutronium, strongly symmetric matter, weakly symmetric matter, quarkgluon plasma, fermionic condensate, Bose-Einstein condensate and finally... Strange matter (1).…………………………………………………………………………………….. …State of Matter" is not the same as "Matter" therefore a correct answer to this question would be (1) All matter comes in many states, a single atom is an atom, a single molecule is a molecule. (1) The differing arrangements of atoms or molecules are what make up the various states of matter it can fulfil. (1) ……………………………………………………………….. …………………………………………………………………………………… (4)