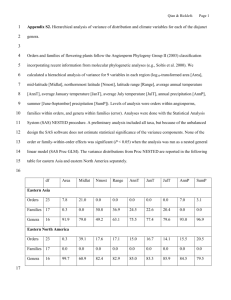
The main biological and zoogeographical characteristics of these
advertisement

Annex 3. The main biological and zoogeographical characteristics of these water dependant taxa can be shortly described as follows: 1- The Pauliniidae (two monospecific genera, Paulinia and Marellia) are linked to water.They are highly polymorphic relatively to their coloration and wing development and have been described under various species names, now synonymised. They range over the whole warm temperate and tropical areas of South America, east to the Andes. Paulinia nevertheless reaches Panama (Carbonell, 1982). Paulinia acuminata completes its entire life cycle on aquatic macrophytes of sheltered waters and is specialised on Salviniaceae (different species of Salvinia and Azolla), but can also feed on the Araceae Pistia stratiotes and Hydromystria stolonifera (Hydrocharitaceae). Oothecas are epiphylle and deposited under the water surface. The link of the ootheca with the aquatic fern is absolute. The genus is fundamentally sedentary, but, during the dry season with shallow waters, when macrophytes disappear, the last suitable places with remnant water and vegetation gather an important density of individuals (to more than 40 specimens / m2) after what they disappear; there is thus a necessity of recolonization which takes place by both active (night flights of macropterous forms) or passive means (oothecas transportation on the floating Salviniaceae at high waters). P. acuminata has been introduced in different places of the world. From East Africa it has now expanded towards the tropical zone (Mozambique (Carbonell, 1981), Botswana (Bennett, 1977), but permanent implantation remains problematic. Details of the life cycles and biology are given for central Amazonia and for the southern region in (Carbonell, 1964; Thomas, 1980; Vieira & Adis, 1992; Vieira & Adis, 2000) Marellia remipes lives on aquatic plants with large leaves floating horizontally on the surface of water: Hydrocleis nymphoides (Butomaceae) and Hydromystria stolonifera (Hydrocharitaceae); egg pods are laid, as in Paulinia at the undersurface of the floating leaves, and the strength of the link between the egg pod and the plant is also important (Carbonell & Arrillaga 1959). This species does not seem to have the same density as P. acuminata, but it may be due only to insufficient knowledge. General distribution and dispersal / recolonisation modalities are similar to Paulinia. It is not known to occur west of the Andes. 2 - The Acrididae, with two subfamilies, the Leptysminae and the Copiocerinae. A- Leptysminae Contrarily to the Pauliniidae, the Leptysminae has a wider distribution east and west of the Andes. Their two tribes (Tetrataeniini and Leptysmini), which include a set of genera completely to more or less linked to fresh water environment occupy this ecosystem in different ways. The first tribe, the Tetrataeniini (more characteristic of forest biota (Amédégnato, 1977)) presents diverse adaptations and produced the genus Cornops, one of the most characteristic sub-aquatic genus of South America. For this tribe, 7 genera and 12 species out of the 11 genera and 40 species known are involved (Roberts & Carbonell, 1979; Roberts & Carbonell, 1980). Two genera (4 species) are strictly linked to aquatic ecosystems: Haroldgrantia (1 known species: H. lignosa) and Cornops (3 of its 4 species). The other 5 genera (6 species) are only linked to damp marshy zones. Some widely distributed species are more characteristic of the riverside in Monocotyledons rich biota (Musaceae, Marantaceae), not obligatory in the inundation zone, but always in humid places. It is the case of the common Tetrataenia surinama and Stenopola boliviana. Tetrataenia is nevertheless often found feeding on Eichhornia with Cornops. It is often believed that most of the Tetrataeniini are linked to water. However, our personal observations indicate that this is not true. In fact, the rest of the tribe has wider possibilities or even has a different ecology (genus Nadiacris, most of the species of Stenopola, except, in part, S. pallida, and all the species of Chloropseutes which live in forest clearings (Amédégnato, 2003)). For most of the genera, few is known about the reproduction. However, when known, the oviposition is endophytic. As all the genera have the same type of cutting ovipositor valves, this mode is probably general. Haroldgrantia lignosa is the only Tetrataenini with a divergent graminicolous morphology. It is linked to aquatic biota, especially to the flooded margins of marshes, with Typha, where egg laying takes place; the genus is actually very poorly known, and only from the south of the neotropical zone (Argentina to Brasil). It is bivoltine and also feeds on other plants (Turk & Aquino, 1995). Cornops There are 4 known species of Cornops. All the species of the genus have an ovipositor specialised to rasp the surface of the stem and cut the bark of their host plant. Eggs are deposited into the parenchyma. Nymphs develop on the host plant; they can easily be identified through their characteristic coloration (green with red stripes), and do not disperse until the end of their development. Distribution data are given in (Adis et al., 2006; Roberts & Carbonell, 1979). One of the species of the genus (C. frenatum) retains the original behaviour of the tribe, and is terrestrial (living on Monocotyledones of the genera Heliconia and Canna). It can usually be collected on forests edges, far enough from water (Amédégnato , 2003), but it is often misidentified with C. aquaticum, when closely parapatric. The remaining of the genus are markedly aquatic: * Cornops brevipenne, which has been described from marshes, and since its description has been found again only very recently and only in five other very distant localities; the species is thus actually known from the amazonian Andean foot, from Bolivia to Ecuador and to east of western Amazonia of Peru and south Colombia (personal collection studies, BMNH and MNHN) until Brasil (Manaus and Parana) ; this means that its distribution area is largely unknown; its host plant, Pontederia rotundifolia (Pontederiaceae) is also of recent discovery (Braga & Adis, 2007). * Cornops aquaticum which is the most aquatic species of the genus; it is also the only one which is found in the tropical zone of Central America as far as Mexico, this being probably related to its high dispersal abilities. It is being studied in view to be released elsewhere in the world to control aquatic weeds (Hill & Oberholzer, 2000; Hill & Olckers, 2000 ( 2001)). The biology of the species is relatively well known, due to its economic importance. However, in different studies of applied entomology some observations may not be accurate due to misidentifications of some closely related species of Cornops that live in sympatry. Besides, the references to “C. longicorne”, or to “C. longipenne” may be errors of misidentification with C. aquaticum. Cornops aquaticum is linked to Eichhornia, mainly E. crassipes and E. azurea,but also to Pontederia spp, all Pontederiaceae; Egg laying takes place in the thick stems of the plant or in the leaf petiole, out of water. The species is bivoltine in Central Amazonia and has a relatively high reproductive potential for the group (30 to 70 eggs / ootheca). The density of the insects may be important enough to cause severe damages and disappearance of mats. Details of the biology are given mainly in : Adis & Junk, 2003; Adis et al., 2004; Ferreira & Vasconcelos-Neto, 2001; Guido & Perkins, 1974; Hill & Oberholzer, 2000; Lhano et al., 2005; Oberholzer & Hill, 2001; Vieira & Santos, 2003; Zolessi, 1956. * Cornops paraguayense which is frequently found with C. aquaticum, from Argentina to Guyana and Venezuela, through central Amazonia, but is actually unknown from western Amazonia (the most rainy parts of the basin); The species is univoltin and presents a very long diapause, which is characteristics of savannah ecosystems, that seem to be the species preferendum. Typha is the main host plant, at least for egg laying (Turk & Aquino, 1996), but C. paraguayense is also found, like C. aquaticum on floating Water Hyacinths. C. paraguayense lives in French Guyana with Leptysmini : Leptysma (L. filicornis or L. intermedia, depending on the locality), Belosacris coccineipes and Stenacris xanthochlora. In the south of the neotropical region, it shares the Typha habitat with Harolgrantia lignosa (Tetrataeniini) and Leptysmina gracilis (Leptysmini). The second tribe, the Leptysmini (Amédégnato, 1974; Amédégnato, 1977; Roberts, 1975; Roberts 1978), with 6 genera and 27 species involved, is entirely graminicolous and graminivorous, all the species living in the inundation zones, except for some species of the genus Cylindrotettix adapted to dry savannah (3-4 species on 13). Two genera are of unknown ecology (total: 8 genera, 33 species). The species have endophytic egg pods in the stems of Gramineae (Hilliard, 1982; Nunes & Adis, 1992; Turk & Aquino, 1998). Being linked to quasi permanently flooded places, they respond to the hydrologic cycle for their reproduction and are fundamental at the basis of the trophic chain in the large surfaces of the flood plains lakes. Stenacris (6 species), Tucayaca (4 species), Belosacris (2 species), and Leptysma (5 species) are sympatric and form the usual association. These genera range over the whole neotropical region, with vicariance of species and subspecies depending on the biogeographic subregion of South and Central America. Stenacris, Leptysma and Belosacris reach the south east of the USA. Tucayaca seems to be more specific from the south but nevertheless reaches Central America. Stenacris is the most abundant and dominates during high water (10 to 100 individuals per m2) while Tucayaca is more abundant during low water (4 to 25-35 individuals / m2 ). Belosacris, while present everywhere, is rarely mentioned, as the least important in density. Leptysma, on the contrary is abundant, but often found in grasses towards the periphery, and may also live in drier grasses, like Cylindrotettix, a genus with some species adapted to tall grasses in dry areas. Stenacris and Tucayaca live on Paspalum repens and Elaeocharis sp. ; egg pods are deposited mainly within Paspalum repens and 4 other Poaceae, (notably Oryza perennis), but also in Eichhornia crassipes. Data are given for central Amazonia (Nunes & Adis, 1994; Nunes et al., 1992). Leptysmina (3 species, all in the southern part of the neotropical region): only Leptysmina gracilis is known and seems to be restricted to Typha or high graminae and Cyperaceae. It is bivoltine (Turk & Aquino, 1998). B - The Copiocerinae (Amédégnato,1977; Descamps, 1984), the second subfamily of the Acrididae, has its main group (Copiocerae of the principal tribe Copiocerini), linked to the palm trees (8 genera on the 10 known); it is, by this way, linked to Varzea and Igapo, mainly to Euterpe. Are especially known for these biota the genera Eumecacris (9 known species) and two close genera: Copiocera and Copiocerina gathering 18 known species. In guyanan Amazonia (Descamps 1978), as well as in western Amazonia (unpublished personal observations and report), local populations comprise 1 or 2 species of Eumecacris (Amazonian realm only, with local endemic species) and 3 species of Copiocera / Copiocerina, in general one of wide distribution, the other local. All species have an epiphylle egg laying on their host plant. This group is very diverse, completely endemic to the neotropics and highly characteristic of neotropical riverine associations. It is more diversified in the southern border of the Amazon basin, where tropical humid forests meet the Cerrado zone (Descamps 1978; Amédégnato, report: "L'acridofaune palmicole néotropicale: diversité et origine"), but reaches Central America with few species. However, it is not known if these species participate there in riverside associations. The group being of recent discovery, it is still not well known. C - Other groups: Besides these main constituents of the fresh water biota, damp riverine associations also have some isolated members of phyla, mainly adapted to other biota, which are marginally adapted there. It is the case for 2 genera of the Rhytidochrotinae (a neotropical mountain bush subfamily): Talamancris palustris in Costa Rica and Palandella cardinalis in south northern Andes of Ecuador. The other genera and species cited are opportunists elements of large continental families like the Romaleidae Coryacris angustipennis in tropical southern south America (COPR, 1982) and Romalea microptera in north America (Squitier & Capinera, 2002). References: Adis, J. & W. Junk, 2003. Feeding impact and bionomics of the grasshopper Cornops aquaticum on the water hyacinth Eichhornia crassipes in Central Amazonian floodplains. Studies on Neotropical Fauna and Environment 38: 245-249. Adis, J., E. Bustorf, M.G. Lhano, C. Amédégnato & A. L. Nunes, 2007. Distribution of Cornops grasshoppers (Leptysminae: Acrididae: Orthoptera) in Latin America and the Caribbean Islands. Studies on Neotropical Fauna and Environment 41:11-24. Adis, J., M. Lhano, M. Hill, W.J. Junk, M.I. Marques & H. Oberholzer, 2004. What determines the number of juvenile instars in the tropical grasshopper Cornops aquaticum (Leptysminae : Acrididae : Orthoptera) ? Studies on Neotropical Fauna and Environment 39 : 127-132. Braga (de S.), C.E. & J. Adis, 2007. Pontederia rotundifolia (Pontederiaceae): host plant of Cornops brevipenne (Leptysminae: Acrididae: Orthoptera). Amazoniana 19 (3/4) : 225. Amédégnato, C., 1974. Les genres d'acridiens néotropicaux, leur classification par familles, sous familles et tribus. Acrida 3 : 113-204. Amédégnato, C., 1977. Etude des Acridoidea centre et sud américains (Catantopinae sensu lato), Anatomie des génitalia, classification, répartition, phylogénie. Universite Pierre et Marie Curie, Paris : 385 pp. Amédégnato, C., 2003. Microhabitat distribution of forest grasshoppers in the Amazon. In: Basset, Y., Novotny, V., Miller, S.E., Kitching, R. (Eds.), "Arthropods of Tropical Forests: Spatio-Temporal Dynamics and Resource Use in the Canopy". Cambridge University Press: 237-255. Carbonell, C.S., 1964. Habitat, ecologia y ontogenia de Paulinia acuminata (DG.) (Acridoidea, Pauliniidae) en el Uruguay. Revista de la Sociedad Uruguaya de Entomologia 6: 39-48. Carbonell, C.S., 1981. Orthoptera. in S.H.Hurlbert, G. Rodriguez & N.Dias dos Santos (eds.) Aquatic biota of tropical South America. Part 1, Arthropoda, San Diego, Calif. 323 pp.: 92-99. Carbonell, C.S., 1982. Orthoptera. in S.H.Hurlbert & A.Villalobos-Figueroa (eds.) Aquatic biota of Mexico, Central America and the West Indies, 529 pp., San Diego, Calif. 529 pp.: 283-287. Carbonell, C.S. & B. Arrillaga, 1959. Sobre la relacion anatomica de las ootecas de Marellia remipes Uvarov (Orthoptera, Acrid. Pauliniidae) con las hojas de su planta huasped, y su posible significacion fisiologica. Revista de la Sociedad Uruguaya de Entomologia 3: 45-56. COPR, 1982. Centre for Overseas Pest Research: The Locust and Grasshopper Agricultural Manual: 690 pp. Descamps, M., 1978. Etude des écosystèmes guyanais, III. Acridomorpha dendrophiles (Orthoptera Caelifera). Annales de la Société Entomologique de France (N.S.) 14 : 301-349. Descamps, M., 1984. Revue préliminaire de la tribu des Copiocerini (Orth. Acrididae). Mémoires du Muséum National d'Histoire Naturelle, Paris, Sér. A, Zoologie 130 : 172. Ferreira, A.S. & J. Vasconcelos-Neto, 2001. Host plants of the grasshopper Cornops aquaticum (Bruner) (Orthoptera: Acrididae) in the wetland of Poconé, MT, Brazil Neotropical Entomology 30: 523-533. Guido, S.A. & B.D. Perkins, 1974. Biology and host specificity of Cornops aquaticum (Bruner) (Orthoptera: Acrididae), a potential biological control agent for water hyacinth. Environemental Entomology 4: 400-404. Hill, M.P. & I.G. Oberholzer, 2000. Host-specificity of the grasshopper Cornops aquaticum, a natural enemy of water hyacinth. In: Spencer, N.R. (Ed.), Proceedings of the 10th International Symposium on Biological Control of Weeds: 349-356. Hill, M.P. & T.B. Olckers, 2001. Biological control initiatives against water hyacinth in South Africa: constraining factors, success and new courses of action. In: Biological and Integrated control of water hyacinth, Eichhornia crassipes. In: Julien, M.H., M.P. Hill, T.D. Center & J.Ding (Eds), Proceedings of the Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Beijing, China, 9-12 October 2000. Australian Centre for International Agricultural Research,Camberra, Australia: 33-38. Hilliard, J.R., 1982. Endophytic oviposition by Leptysma marginicollis marginicollis and Stenacris vitreipennis (Orthoptera: Acrididae: Leptysminae) with life history notes. Transactions of the American Entomological Society 108: 153-180. Lhano, M.G., J. Adis, M.I. Marques & L.D. Battirola, 2005. Cornops aquaticum (Orthoptera, Acrididae, Leptysminae): aceitação de plantas alimentares por ninfas vivendo em Eichhornia azurea (Pontederiaceae) no Pantanal Norte, Brasil. Amazoniana 18: 397-404. Nunes, A.L. & J. Adis, 1992. Observaciones sobre el comportamiento sexual y la oviposicion de Stenacris fissicauda fissicauda (Bruner, 1908) (Orthoptera, Acrididae). Etologia 2: 59-63. Nunes, A.L. & J. Adis, 1994. Comportamento populacional de Tucayaca gracilis (Giglio-Tos, 1897) (Orthoptera - Acrididae) frente a oscilaçao do nivel d'agua na varzea da Amazonia Central. Boletim do Museu paraense Emilio Goeldi, Serie Zoologia 10: 211-224. Nunes, A.L., J. Adis & J.A.S. Nunes De Mello, 1992. Estudo sobre o ciclo de vida e fenologia de Stenacris f. fissicauda (Bruner 1908 )(Orthoptera : Acrididae) em um lago de varzea da Amazonia Central, Brasil. Boletim do Museu paraense Emilio Goeldi, Serie Zoologia 8: 349-374. Roberts, H.R., 1975. A revision of the genus Cylindrotettix including new species (Orthoptera; Acrididae; Leptysminae). Proceedings of the Academy of Natural Sciences of Philadelphia 127: 29-43. Roberts, H.R., 1978. A revision of the tribe Leptysmini except the genus Cylindrotettix (Orthoptera: Acrididae: Leptysminae). Proceedings of the Academy of Natural Sciences of Philadelphia 129: 33-69. Roberts, H.R. & C.S. Carbonell, 1979. A revision of the genera Stenopola and Cornops (Orthoptera, Acrididae, Leptysminae). Proceedings of the Academy of Natural Sciences of Philadelphia 131: 104- 130. Roberts, H.R. & C.S. Carbonell, 1980. Concluding revision of the subfamily Leptysminae (Orthoptera, Acrididae). Proceedings of the Academy of Natural Sciences of Philadelphia 132: 64-85. Squitier, J.M., Capinera, J.L., 2002. Habitat associations of Florida grasshoppers (Orthoptera: Acrididae). Florida Entomologist 85: 235-244. Thomas, P.A., 1980. Life cycle studies on Paulinia acuminata (De Geer ) (Orthoptera: Pauliniidae) with particular reference to the effects of constant temperature. Bulletin of Entomological Research 70: 381-389. Turk, S.Z. & A.L. Aquino, 1995. ACRIDOIDEOS DEL N.O.A. VII : Estudios bioecológicos en Haroldgrantia lignosa Carbonell, Ronderos y Mesa (Acrididae:Leptysminae: Tetrataeniini).Un nuevo caso de oviposición endofítica en el noroeste argentino. Acta Zoologica Lilloana 43: 99-103. Turk, S.Z. & A.L. Aquino, 1996. ACRIDOIDEOS DEL N.O.A. VIII : Nuevo aporte a la bioecología y disribución del género Cornops Stal: Cornops paraguayense (Br.). (Acrididae: Leptysminae:Tetrataeniini). Acta Zoologica Lilloana 43: 427-432. Turk, S.Z. & A.L. Aquino, 1998. ACRIDOIDEOS DEL N.O.A. IX : Contribución al conocimiento de los Leptysminae Neotropicales : Ciclo de vida de Leptysmina gracilis Bruner (Acrididae: Leptysminae: Leptysmini). Acta Zoologica Lilloana 44: 185-190. Vieira, M.d.F. & J. Adis, 1992. Abundancia e biomassa de Paulinia acuminata (De Geer,1773) (Orthoptera, Pauliniidae) em un lago de varzea da Amazônia Central. Amazonia 12: 337-352. Vieira, M.d.F. & J. Adis, 2000. Biological and ethological aspects of the semi-aquatic grasshopper Paulinia acuminata (De Geer), 1773 (Orthoptera: Pauliniidae) in Central Amazonia, Manaus, Brazil. Acta Amazonica 30: 333-346. Vieira, M.d.F.& A.C.d Santos, 2003. Duração do ciclo de vida de Cornops aquaticum (Bruner, 1906) (Orthoptera: Acrididae: Leptysminae) e aspectos de seu comportamento alimentar na Amazônia Central. Acta Amazônica 33: 711-714. Zolessi, L.C., 1956. Observaciones sobre Cornops aquaticum Br. (Acridoidea, Cyrtacanthacr.) en el Uruguay. Revista de la Sociedad Uruguaya de Entomologia 1: 3-28.