PA 54 Polar bonds and electronegativity Printable
advertisement

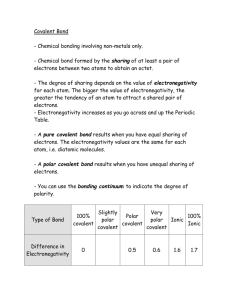
Prescriptive Activities: Facet Cluster 1.6: PF#54 Teacher Page Prescriptive #54: 54 The student does not use the periodic table to accurately predict polar bonds. *Note: Misconception found is that in all covalent bonds electron pairs were shared equally Materials: Student handout Internet access Background: When nonmetal atoms bond together to form covalent compounds, they will either share the electrons equally between the atoms, or the electrons will be more attracted to one atom than another. This unequal attraction results in a polar bond and is very important in determining the properties of substance. Electronegativity is defined as the ability of an atom in a molecule to attract shared electrons toward itself. This ability depends on the nuclear charge (number of protons) and the distance of the outermost electrons from the nucleus. Electronegativity values have been experimentally determined and can be found in the table provided on the next page. In a covalent bond, the more electronegative atom will attract the shared electrons more strongly towards itself and away from the less electronegative atom. (Remember, covalent bonds share electrons, while ionic bonds do not) This unequal sharing will create more “electron density” or partial negative charge near the more electronegative atom, as shown for HF at right. Fluorine is more electronegative than hydrogen, and so the shared electrons spend more time around the fluorine atom. The arrow is pointing towards the slightly negative side of the molecule. We say that this more electronegative side of the bond has a partial negative charge, symbolized by the lowercase Greek letter delta (δ-). The side of the bond with the less electronegative atom has a partially positive charge, symbolized by “δ+”. H H If two atoms have the same electronegativity then they both attract shared electrons equally and the bond is a nonpolar covalent bond. The hydrogen (H2) molecule shown at left is an example of a nonpolar molecule. 1 Prescriptive Activities: Facet Cluster 1.6: PF#54 Teacher Page To determine if a bond is polar or nonpolar, you look at the electronegativity values of the two atoms (shown on the periodic table below). If the numbers are equal, or their difference is under .5, they are nonpolar. If the numbers are not equal or under .5, then the atom with the higher electronegative value has a partial negative charge If the atoms have a large difference in electronegativity, more than 1.6, the bond is an ionic bond and they do not share electrons. Note: The difference in electronegativity is symbolized by EN Electronegativity difference 0-0.4 0.5-1.6 1.7 or more Bond type Nonpolar covalent Polar covalent Ionic One example of a polar covalent bond is the Si-C bond. The electronegativity difference between silicon and carbon is 2.5-1.8 = 0.7 (ΔEN = 0.7). From the table above you can see that this would create a polar covalent bond. Because carbon (2.5) is more electronegative than silicon (1.8), the carbon side of the bond would have a partially negative charge and the silicon side would have a partially positive charge. This is diagrammed below. δ+ Si C δ- 2 Prescriptive Activities: Facet Cluster 1.6: PF#54 Teacher Page Electronegativity Table Directions of Activity: Watch the video about electronegativity located here and then answer the questions on the next page: Title: Electronegativity (1.25) http://www.youtube.com/watch?v=Kj3o0XvhVqQ&feat ure=player_detailpage Note: You may notice that the value ranges given in the video for ionic, polar and nonpolar bonds are slightly different than those given in this activity. It is difficult to make an absolute distinction between these types of bonds and therefore you will find that different scientists, books and resources will give different values. This is acceptable as long as you understand that there is a continuum that exists in going from nonpolar to polar to ionic bonds and this is determined by the ability of an atom to attract electrons to itself within a bond. Table I Bond H-N Nonpolar Polar Ionic H-C H-F N-O K-S S-F F-F Mg-Cl H-H Br-Cl Check with your teacher to find out which values you are expected to use in class. 3 Prescriptive Activities: Facet Cluster 1.6: PF#54 Teacher Page Questions: 1. Use the electronegativity table to determine if each of the bonds shown in Table I is polar covalent, nonpolar covalent or ionic. 2. Use the electronegativity table to determine which side of the bond will be slightly negative and which side slightly positive in a covalent bond between the pairs of nonmetals shown below. Circle the atom that will have the partial negative charge. a. P-Cl b. H-Cl c. O-S d. O-F 3. Why are no electronegativity values shown for the noble gases? Teacher Notes: The students can do this activity without watching the video about electronegativity. The video provide a dynamic visual to help students understand the concept, and it includes a good explanation. A note about the continuum of bond types: There are many opinions on where to draw the line between polar covalent bonds and ionic bonds. Here’s one option for resolving this issue. You can consider 0.5-1.6 to be polar covalent, and > 1.9 ionic. Between 1.7 and 1.9, inclusive, it is polar covalent if the bond is between two nonmetals, and ionic if the bond involves a metal. References: Electronegativity Table from: http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/Resonance Video: Electronegativity, http://www.youtube.com/watch?v=Kj3o0XvhVqQ Computer animation about nonpolar, polar and ionic bonds 4 Prescriptive Activities: Facet Cluster 1.6: PF#54 Teacher Page HF molecule image from: http://darkwing.uoregon.edu/~ch111/L13.htm Answer Key Question 1: Table I Bond H-N Nonpolar H-C X Polar X H-F X N-O X K-S Ionic a. P-Cl X S-F Question 2: Use the electronegativity table to determine which side of the bond will be slightly negative and which side slightly positive in a covalent bond between the pairs of nonmetals shown below. Circle the atom that will have the partial negative charge. b. H-Cl c. O-S X d. O-F F-F X Mg-Cl X H-H X Br-Cl X Question 3: The noble gases do not tend to form bonds because they have a full valence energy level and are very stable. Therefore, they do share electrons and thus do not have electronegativity values. 5