SOP_for_Autoclave_Located_in_the_Isolation_Facility
advertisement

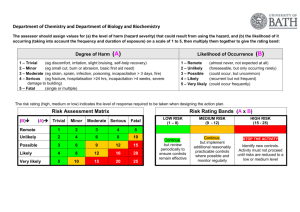
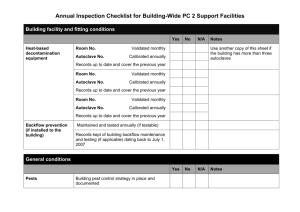
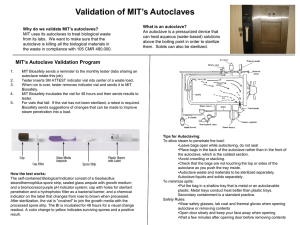
Standard Operating Procedures for Autoclave Located in the Isolation Facility 1. The following personnel are approved to operate this equipment: Sarah Gorsky Debbie Masciulli Shawn Davis Lionel Snyder Betsy Snyder 2. Bedding and byproducts from patients determined to be positive for Salmonella, Cryptosporidium and MRSA cases will require processing through the autoclave located in the Isolation facility. 3. Using Isolation Cleaning and Disinfecting (C&D) protocols, the stall should be cleaned and bedding and by products bagged in autoclavable bags. Autoclave bags are located in Isolation Room 119 Care should be given when filling the bags to not overfill. After closing the top of the bag load onto the autoclave cart taking care not to tear the bag. Load bags in a fashion that will allow steam circulation around all areas of the bag. Two to three bags is recommended per load. Additional bags can be stored in a yellow cart until autoclaved. 4. Heat resistant gloves should be worn when removing cart from the autoclave. Gloves are located on top of autoclave. Never open the autoclave door until the run is finished. Use extreme caution when opening the autoclave door. Remove bags from cart and place in black plastic bags prior to transporting to bovine dumpster. IN CASE OF MACHINE OPERATIONAL EMERGENCY CONTACT: Terry Robertson, 541-760-8842 FOR REPAIRS CONTACT: Terry Robertson, 541-760-8842 Lionel Snyder, 541-224-4051, Monday-Friday, 8 a.m.-5 p.m. THIS AUTOCLAVE IS INTENDED SOLELY FOR THE USE IN THE DECONTAMINATION OF BIOHAZARD WASTE GENERATED BY ISOLATION PATIENTS 7/5/11 PH, LS