Biological Waste Disposal
advertisement
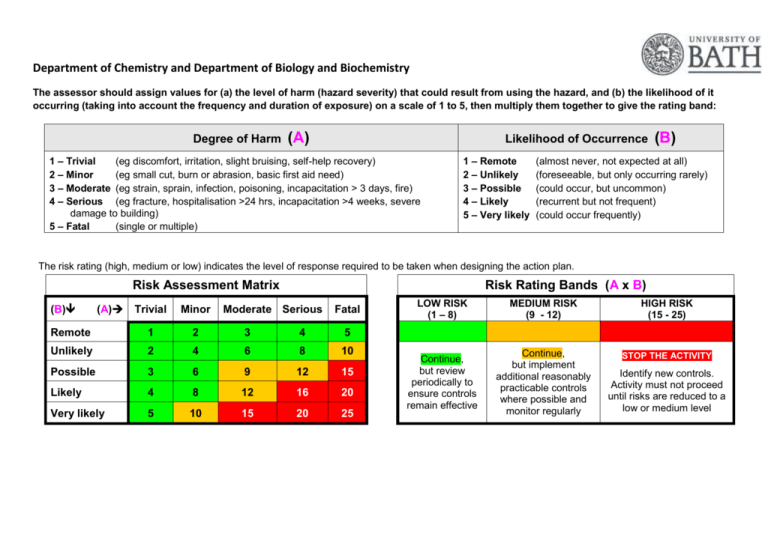
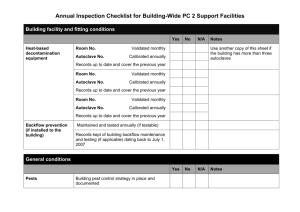
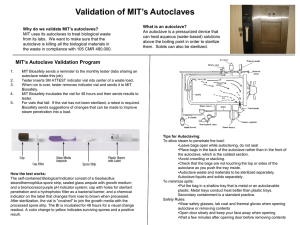
Department of Chemistry and Department of Biology and Biochemistry The assessor should assign values for (a) the level of harm (hazard severity) that could result from using the hazard, and (b) the likelihood of it occurring (taking into account the frequency and duration of exposure) on a scale of 1 to 5, then multiply them together to give the rating band: Degree of Harm (A) Likelihood of Occurrence 1 – Trivial (eg discomfort, irritation, slight bruising, self-help recovery) 2 – Minor (eg small cut, burn or abrasion, basic first aid need) 3 – Moderate (eg strain, sprain, infection, poisoning, incapacitation > 3 days, fire) 4 – Serious (eg fracture, hospitalisation >24 hrs, incapacitation >4 weeks, severe damage to building) 5 – Fatal (single or multiple) 1 – Remote 2 – Unlikely 3 – Possible 4 – Likely 5 – Very likely (B) (almost never, not expected at all) (foreseeable, but only occurring rarely) (could occur, but uncommon) (recurrent but not frequent) (could occur frequently) The risk rating (high, medium or low) indicates the level of response required to be taken when designing the action plan. Risk Assessment Matrix (B) (A) Risk Rating Bands (A x B) Trivial Minor Moderate Serious Fatal Remote 1 2 3 4 5 Unlikely 2 4 6 8 10 Possible 3 6 9 12 15 Likely 4 8 12 16 20 Very likely 5 10 15 20 25 LOW RISK (1 – 8) MEDIUM RISK (9 - 12) HIGH RISK (15 - 25) Continue, but review periodically to ensure controls remain effective Continue, but implement additional reasonably practicable controls where possible and monitor regularly STOP THE ACTIVITY Identify new controls. Activity must not proceed until risks are reduced to a low or medium level Risk Assessment of: Disposing of biological waste. Assessor(s): Marianne Harkins Work Authorised by: Date: Jan 2015 Overview of activity / location / equipment / conditions being assessed: A risk assessment of how to dispose of biological waste in 1 South, 3 South and 4 South. How biological waste is sorted and categorised before being autoclaved. Note: Staff may contact the technical team who deal with biological waste on bbcwaste@lists.bath.ac.uk. Definitions: A biological agent: ‘a micro-organism, cell culture, or human endoparasite, whether or not genetically modified, which may cause infection, allergy, toxicity or otherwise create a hazard to human health.’ Most biological agents are micro-organisms, ie bacteria, viruses, fungi. Microbial endoparasites such as the malarial parasite, amoebae and trypanosomes and the microscopic forms of the larger endoparasites such as the ova and larval forms of helminths. A micro-organism is defined in COSHH ‘a microbiological entity, cellular or non-cellular, which is capable of replication or of transferring genetic material.’ Decontamination: ‘the process whereby microbial contamination of a material is reduced to render it safe to handle. There are two methods of achieving this, disinfection and sterilisation, which should not be confused’. Disinfection refers to a treatment that is designed to reduce the potential infectivity of a material to a level that effectively destroys its potential to cause harm. It does not necessarily remove all viable micro-organisms which is instead the aim of a sterilisation process. The choice of which decontamination method to use in any particular situation will depend upon the level of decontamination required and should be determined by PIs in their activity risk assessment. Hazard Who could be harmed? Control measures needed to minimise risk General Laboratory users, staff, support staff, PG and UG students, contractors Anyone working in the Department will have a health and safety induction. Staff are informed about how to deal with biological waste. All work will be Risk Assessed. At a minimum a lab coat and safety glasses should be worn in the store at all times. Any PPE specified for working with specific chemicals should be word/ used. Risk after controls implemented A B AxB 2 2 4 Display suitable and sufficient warning signs, for example, displaying the containment level (CL), including the biohazard sign. Use appropriate decontamination (see definition above) procedures. Contaminated surfaces Environmental/ human health impacts resulting in illness, infection or disease. Contaminated waste Environmental/ human health impacts resulting in illness, infection or disease. All surfaces (including lab benches, laminar flows, fume hoods and any other surface where biological work is carried out) should be decontaminated with vircon, bleach or an equivalent decontaminating solution. All cleaning implements should be disposed of in a biological waste autoclave bag. 2 2 4 Biological waste bins are to be used in areas where biological waste may be produced. Autoclave bags are used to line biological waste bins. All biological waste and items (that are not re-useable) potentially contaminated with biological waste should be placed in autoclave bags. Autoclave bags should not be filled above the fill line. No sharps should be placed in autoclave bags. Full autoclave bags should be sealed with autoclave tape (while in the biological waste bin) and taken to the 3 South autoclave room. 2 2 4 Contaminated glassware/ re-useable consumables. Environmental/ human health impacts resulting in illness, infection or disease. Liquid waste should be stored in sealed, autoclave-safe containers. Lids of bottles should be tightened when transferring to the 3 South autoclave room. Lids of bottles should be loosened once stored in the 3 South autoclave room. All waste should be clearly labelled with contents/ details of owner. Autoclave tape should be stuck on all containers and items to be sterilised. 2 2 4 Autoclaving hazardous waste Environmental/ human health impacts resulting Please see the Generic Risk Assessment for Autoclaving. All autoclaved waste is sterilised and is remove to bins outside of 3 South. 2 3 6 and/ or visitors to the laboratory. Waste store and waste compound users. The environment. in illness, infection or disease. Sharps Puncture wounds/ scrapes/ infection. Sharps are to be placed in sharps bins and sealed when the fill-line is reached. A working with sharps Generic RA is available. 2 3 6 Spills/ loss of containment Environmental/ human health impacts resulting in illness, infection or disease. Spills are to be cleaned up as a priority, wearing the correct PPE. Vircon, bleach or similar decontaminating solutions will be used to clean up spills. Safety showers and eyewashes are available in all Departments. A drain cover is available in the 1 South hazardous waste store for when large volumes of water are needed to clear up a spill. 2 2 4 Unauthorised access to facility Access to the department is restricted out of hours. Visitors should be accompanied by an approved person. All keys are kept in a secure place. There is CCTV covering the area and recordings are kept for 14 days before being over-written. Security also regularly patrol the area during the evening and night. 1 5 5 Slips and Trips Slipping on floors/ tripping on hazards Floors are regularly swept top keep them clean. Good house-keeping means that clutter is removed. A waste management technician is employed to ensure best practice. 2 2 4 Manual Handling Musculoskeletal problems. The University Manual Handling guidelines should be followed. 1 2 2