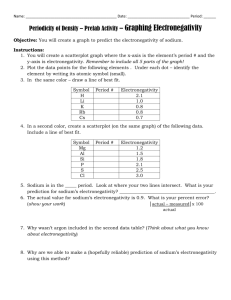
Chemistry Unit 14 Practice Test
advertisement
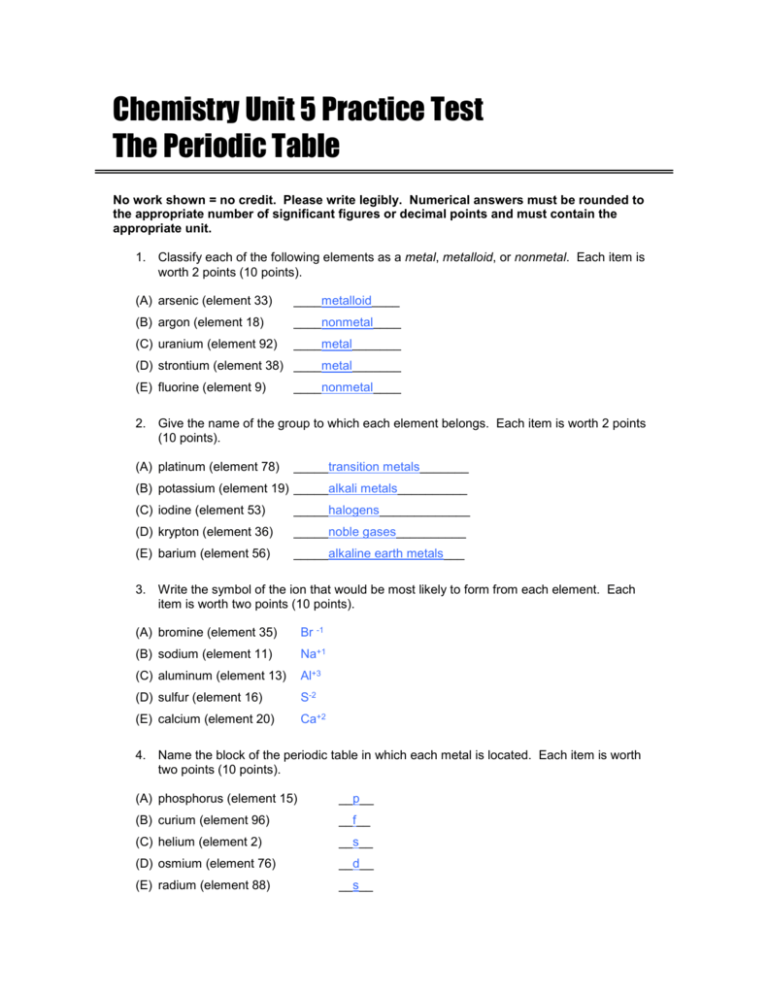
Chemistry Unit 5 Practice Test The Periodic Table No work shown = no credit. Please write legibly. Numerical answers must be rounded to the appropriate number of significant figures or decimal points and must contain the appropriate unit. 1. Classify each of the following elements as a metal, metalloid, or nonmetal. Each item is worth 2 points (10 points). (A) arsenic (element 33) ____metalloid____ (B) argon (element 18) ____nonmetal____ (C) uranium (element 92) ____metal_______ (D) strontium (element 38) ____metal_______ (E) fluorine (element 9) ____nonmetal____ 2. Give the name of the group to which each element belongs. Each item is worth 2 points (10 points). (A) platinum (element 78) _____transition metals_______ (B) potassium (element 19) _____alkali metals__________ (C) iodine (element 53) _____halogens_____________ (D) krypton (element 36) _____noble gases__________ (E) barium (element 56) _____alkaline earth metals___ 3. Write the symbol of the ion that would be most likely to form from each element. Each item is worth two points (10 points). (A) bromine (element 35) Br -1 (B) sodium (element 11) Na+1 (C) aluminum (element 13) Al+3 (D) sulfur (element 16) S-2 (E) calcium (element 20) Ca+2 4. Name the block of the periodic table in which each metal is located. Each item is worth two points (10 points). (A) phosphorus (element 15) __p__ (B) curium (element 96) __f__ (C) helium (element 2) __s__ (D) osmium (element 76) __d__ (E) radium (element 88) __s__ 5. Tell how many energy levels atoms of each element have. Each item is worth 2 points (10 points). (A) tungsten (element 74) __6__ (B) magnesium (element 12) __3__ (C) carbon (element 6) __2__ (D) californium (element 96) __7__ (E) hydrogen (element 1) __1__ 6. Define the phrase ionization energy. (5 points) ionization energy – the energy required to remove 1 electron from an atom. 7. Define the word electronegativity. (5 points) electronegativity – tendency of an atom to attract e- in a chemical bond. 8. Which element has the larger atoms: carbon or oxygen? (5 points) ___C__ 9. Which element has the larger atoms: sodium or potassium? (5 points) ___K__ 10. Which element has the higher ionization energy: sodium or sulfur? (5 points) ___S__ 11. Which element has the higher ionization energy: nitrogen or arsenic? (5 points) ___N__ 12. Which element has the higher electronegativity: silicon or chlorine? (5 points) ___Cl_ 13. Which element has the higher electronegativity: fluorine or chlorine? (5 points) ___F__ 14. TRUE or FALSE: Noble gases have the highest electronegativity values of any elements on the periodic table. Explain your answer. (You do not get full credit for just writing “True” or “False”) (10 points) FALSE. Noble gases have no electronegativity values. The element with the highest electronegativity is fluorine, a halogen.