Periodic Trends Worksheet: Atomic Radius, Ionization Energy
advertisement
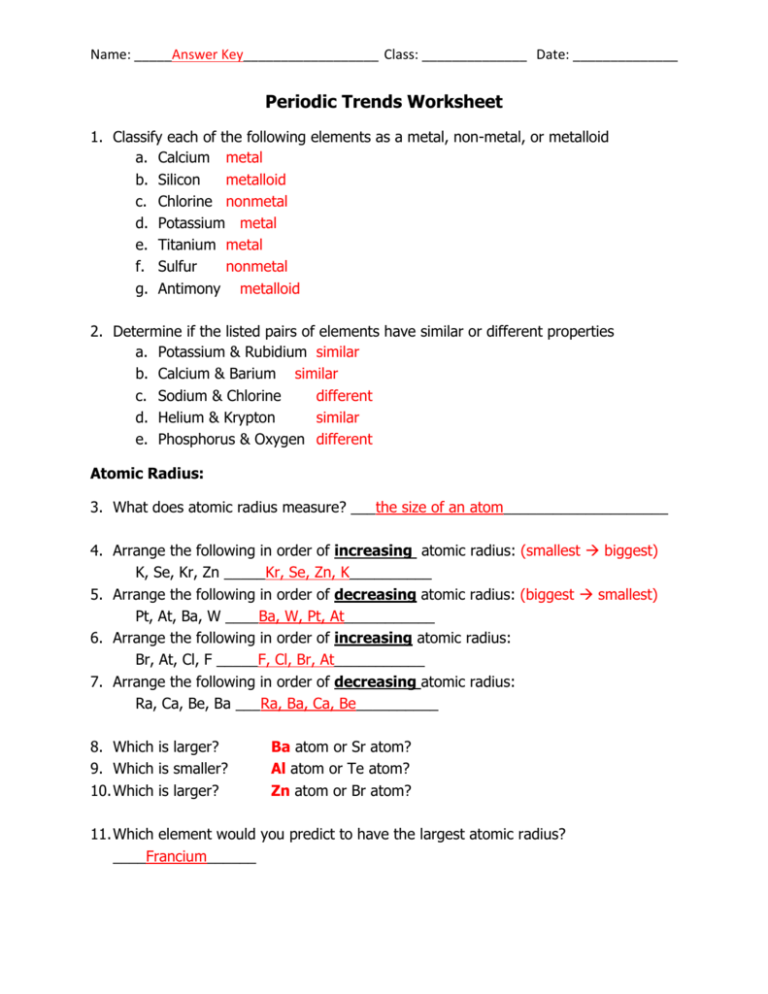
Name: _____Answer Key__________________ Class: ______________ Date: ______________ Periodic Trends Worksheet 1. Classify each of the following elements as a metal, non-metal, or metalloid a. Calcium metal b. Silicon metalloid c. Chlorine nonmetal d. Potassium metal e. Titanium metal f. Sulfur nonmetal g. Antimony metalloid 2. Determine if the listed pairs of elements have similar or different properties a. Potassium & Rubidium similar b. Calcium & Barium similar c. Sodium & Chlorine different d. Helium & Krypton similar e. Phosphorus & Oxygen different Atomic Radius: 3. What does atomic radius measure? ___the size of an atom____________________ 4. Arrange the following in order of increasing atomic radius: (smallest biggest) K, Se, Kr, Zn _____Kr, Se, Zn, K__________ 5. Arrange the following in order of decreasing atomic radius: (biggest smallest) Pt, At, Ba, W ____Ba, W, Pt, At___________ 6. Arrange the following in order of increasing atomic radius: Br, At, Cl, F _____F, Cl, Br, At___________ 7. Arrange the following in order of decreasing atomic radius: Ra, Ca, Be, Ba ___Ra, Ba, Ca, Be__________ 8. Which is larger? 9. Which is smaller? 10. Which is larger? Ba atom or Sr atom? Al atom or Te atom? Zn atom or Br atom? 11. Which element would you predict to have the largest atomic radius? ____Francium______ Ionization Energy: 12. Define ionization energy __the energy required to remove a valence electron___ 13. Which group (name) would have the lowest ionization energies? ___Alkali_Metals________ 14. Which group (name) would have very high ionization energies? ___Noble_Gases_________ 15. Bromine has a higher / lower ionization energy than Lead (circle one) 16. Barium has a higher / lower ionization energy than Radon (circle one) 17. Fluorine has a higher / lower ionization energy than Arsenic (circle one) 18. Lithium has a higher / lower ionization energy than Cesium (circle one) Electronegativity: 19. Define electronegativity __the tendency of an atom to attract electrons to itself____ 20. Which element would you predict to have the greatest electronegativity? ___Fluorine__________ 21. Arrange the following atoms in order of increasing electronegativitiy: Mg, Al, Cl, Si ____Mg, Al, Si, Cl______ (typo At should be Al) 22. Arrange the following in order of decreasing electronegativity: Bi, N, Sb ___N, Sb, Bi_______ 23. Arrange the following in order of increasing electronegativity: S, O, Ne, Al ____Ne, Al, S, O___ 24. Which group (name) would you predict to have the least electronegativity? ___Noble Gases______ Circle the element that is more electronegative: 25. Mg or Sr 26. Be or N 27. P or Cl 28. Ni or Pd











